Mempro™ Cell-Based Peptide Fragments of Transmembrane Protein Prooduction
Creative Biostructure can offer advanced custom Mempro™ peptide fragment of transmembrane protein production services based on cell-based expression systems for further structural analysis. Our seasoned scientists can provide multiple overexpression and purification strategies for high-yield production of transmembrane protein fragments.
Mempro™ cell-based protein production systems are widely used for the production of a large variety integral membrane proteins, such as G-protein coupled proteins (GPCRs). Structural studies of full-length membrane proteins are hindered by the hydrophobicity and low expression of proteins owing to membrane protein folding. Thus, individual transmembrane fragments or segments derived from membrane protein folding have been observed to represent independent folding domains, and as such, can facilitate the study of packing interactions between transmembrane protein helices, and the collection of structural information regarding integral membrane proteins (Figure 1).
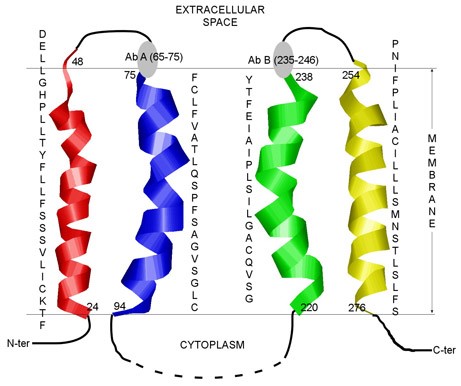 Figure 1. The predicted transmembrane regions of bilitranslocase (BTL). (PLoS ONE, 2012)
Figure 1. The predicted transmembrane regions of bilitranslocase (BTL). (PLoS ONE, 2012)
Creative Biostructure can provide various high-scale strategies for peptide fragments of transmembrane protein production using Mempro™ cell-based proteins expression systems, including:
- Mempro™ Protein Production in Bacterial Cells System ;
- Mempro™ Protein Production in Yeast Cells System;
- Mempro™ Protein Production in Incest Cells System;
- Mempro™ Protein Production in Mammalian Cells System.
Among Mempro™ cell-based protein production platforms, Escherichia coli (E. coli) is used as bacterial host for the production of peptide fragments of transmembrane proteins. Yeast cells (such as saccharomyces cerevisiae and pichia pastoris) system, combines prokaryotic as well as eukaryotic characteristics. Yeast is an attractive eukaryotic host due to single cells, fast growth rates, inexpensive media, high cell densities. Insect cells (such as Sf9 and Sf21) are also a major system for peptide fragments of transmembrane protein expression, which is developed in the early stage that transfected insect cells with the baculovirus species AcMNPV. Mammalian cells present another eukaryotic expression system, which provide the most authentic cell environment. Mammalian cell lines derived from COS, CHO, BHK-21, HEK293, HeLa and GH3 are commonly used for peptide fragments of transmembrane protein production.
Creative Biostructure is capable of expressing, isolating, purifying and solubilizing peptide fragments of transmembrane proteins to facilitate the structural studies. One challenge we should face is the hydrophobic feature of transmembrane peptides, we can overcome this difficulty by ‘tagging’ the hydrophobic fragments termini with cationic residues to mimic native transmembrane segments, as membrane proteins commonly contain residues such as Lys and Arg at membrane entry and exit points.
We provide other various Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
A. Perdih, et al. (2012). Structural analysis of a peptide fragment of transmembrane transporter protein bilitranslocase. PLoS ONE, 7(6): e38967.
F. Cunningham and C. M. Deber (2006). Optimizing synthesis and expression of transmembrane peptides and proteins. Methods, 41(4): 370-380.
J. Venkatraman, et al. (2002). Structural analysis of synthetic peptide fragments from emre, a multidrug resistance protein, in a membrane-mimetic environment. Biochemistry, 41(21): 6631–6639.