Mempro™ Detergent-Free MAPEG (Eicosanoid and Glutathione Metabolism Proteins) Production
Based on the comprehensive protein engineering platform established through years of experience, scientists from Creative Biostructure offer tailored Mempro™ MAPEG (Eicosanoid and Glutathione metabolism proteins) production services using detergent-free expression system.
MAPEG (Membrane-Associated proteins in Eicosanoid and Glutathione metabolism) is a universal protein superfamily which has a set of membrane related proteins with extremely divergent functions. This family is made up of five human proteins: microsomal glutathione S-transferase 1 (MGST1), MGST2, MGST3, MGST1-like 1 (MGST1-L1), and 5-lipoxygenase-activating protein (FLAP). Besides, a few non-mammalian members have been appraised, three are from plants (Oryza sativa, Ricinus communis and Arabidopsis thaliana), one is from fungi (Aspergillus nidulans), and three are from bacteria (Escherichia coli, Vibrio cholerae and Synechocystis sp). The formation of leukotriene A4 (LTA4) from arachidonic acid is catalyzed by 5-Lipoxygenase, and during the reaction leukotrienes acts as a crucial amboceptor. Due to structural comparability in the active sites of FLAP, LTC4 synthase and PGE synthase, substrates for each enzyme can contend with each other and modulate synthetic activity.
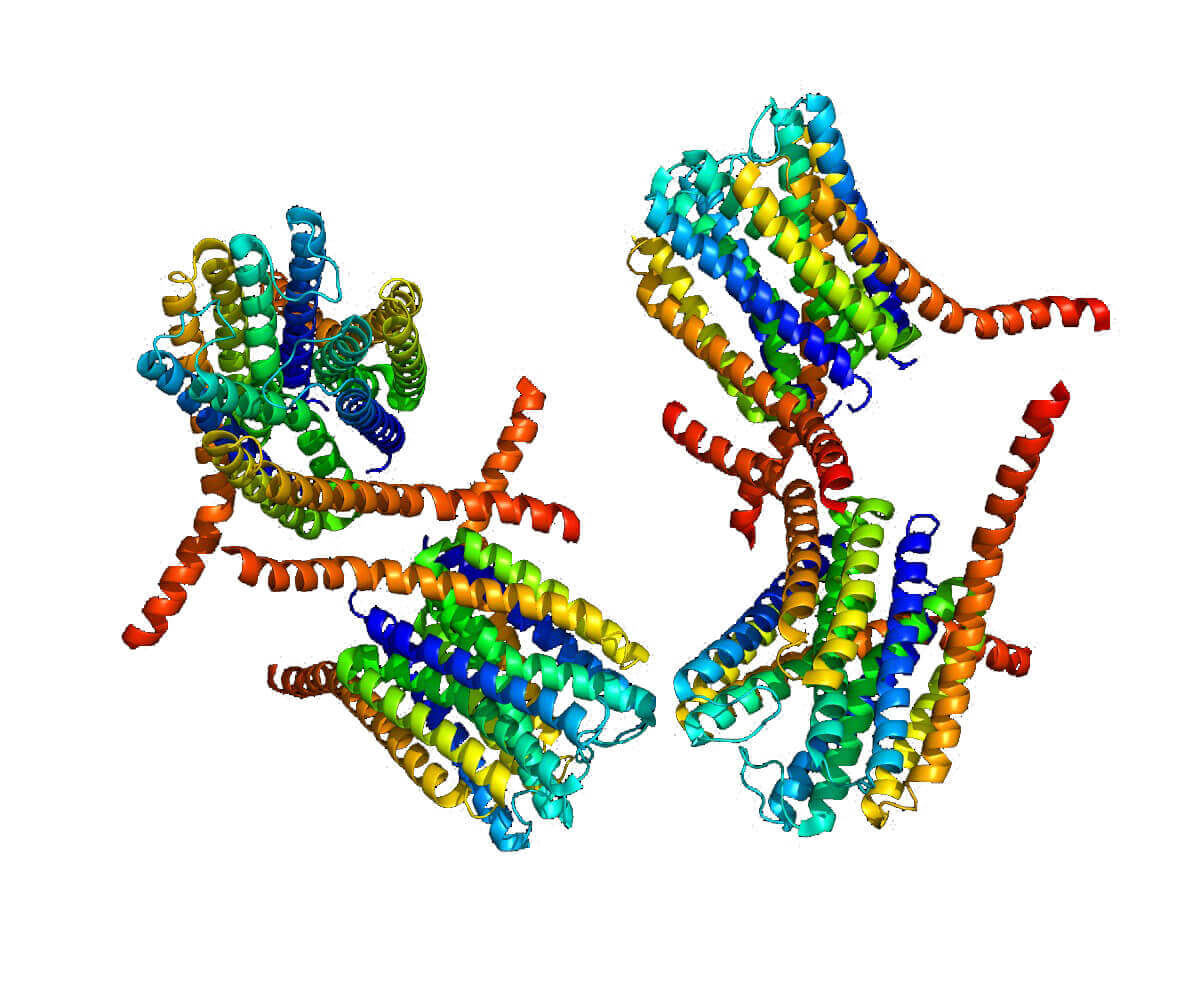
Figure 1. Crystal structure of human leukotrience C4 synthase. (Nature 2007)
Creative Biostructure has rich professional experience in high quality MAPEG production using detergent-free membrane protein expression system, we can perform various strategies for Mempro™ detergent-free protein production, including:
- Mempro™ MAPEG (Eicosanoid and Glutathione Metabolism Proteins) Production Using Nanodiscs
Nanodiscs consist of membrane scaffold proteins (MSPs), which are embellished versions of the major ingredient of HDL, apolipoprotein A1 (apoA1). In contrast with bicelles, detergent micelles and liposomes, a more native environment where membrane proteins remain monodisperse and active can be offered. Various of membrane proteins systems enable to be self-assembled into nanodiscs.
- Mempro™ MAPEG (Eicosanoid and Glutathione Metabolism Proteins) Production Using Amphipols
Amphipols are a class of oligomers and polymers, meanwhile they are portmanteaus of amphiphilic polymers. The wide resources of polymer chemistry can make it possible to tailor the physicochemical properties of amphipols toward peculiar uses in biophysics and biochemistry.
- Mempro™ MAPEG (Eicosanoid and Glutathione Metabolism Proteins) Production Using Poly (styrene-co-maleic acid) Lipid Particles (SMALPs)
Using SMALPs for structure determination of membrane proteins is a novel approach, and with this approach, the gain and speed of protein extraction can be markedly increased. Recently, electron microscopy has quickly progressed, which results in the highly development of data acquisition, together with the conformational and resolution information getable.
These novel detergent-free technologies for MAPEG (Eicosanoid and Glutathione metabolism proteins) production can be obtained easily, and enabling more comprehensively structural and functional studies.
Creative Biostructure provides other various Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
Ago, H., Kanaoka, Y., Irikura, D., Lam, B. K., Shimamura, T., Austen, K. F., & Miyano, M. (2007). Crystal structure of a human membrane protein involved in cysteinyl leukotriene biosynthesis. Nature, 448(7153), 609-612.
Jakobsson, P.-J., Morgenstern, R., Mancini, J., Ford-Hutchinson, A., & Persson, B. (1999). Common structural features of MAPEG—a widespread superfamily of membrane associated proteins with highly divergent functions in eicosanoid and glutathione metabolism. Protein Science, 8(03), 689-692.
Gaczynska, M., Rock, K. L., Spies, T., & Goldberg, A. L. (1994). Peptidase activities of proteasomes are differentially regulated by the major histocompatibility complex-encoded genes for LMP2 and LMP7. Proceedings of the National Academy of Sciences, 91(20), 9213-9217.
Jamshad, M., Lin, Y.-P., Knowles, T. J., Parslow, R. A., Harris, C., Wheatley, M., . . . Overduin, M. (2011). Surfactant-free purification of membrane proteins with intact native membrane environment. Biochemical Society Transactions, 39(3), 813-818.