Mempro™ Detergent-Free Single-helix ATPase Regulators Production
Based on the comprehensive membrane protein production platform establised through years of experience, scientists from Creative Biostructure can offer unparalled custom Mempro™ single-helix ATPase regulators production services using detergent-free expression system.
Single-helix ATPase regulators belong to the bitopic protein superfamily with alpha-helical transmembrane anchors and regulate ATPases. It is well known that alpha-helices and beta-barrels are the main components of the transmembrane domains in transmembrane proteins. Single-helix ATPase regulators can be classified two families, including calcium ATPase regulators and FXYD regulators. Calcium ATPase regulators locate in endoplasm reticulum (ER) and regulate calcium homeostasis. Members of calcium ATPase regulators family include phospholamban, sarcolipin and cytoplasmic helix. FXYD regulators locate in the plasma membrane of eukaryo, members of FXYD regulators family are tissue-specific modulators of Na, K-ATPase. FXYD1 mainly increases the apparent affinity for intracellular Na+ of Na, K-ATPase. FXYD2 and FXYD4 can oppositely regulate the apparent affinity for Na+ of Na, K-ATPase. Conventional approaches for transmembrane protein purification need extraction from the membrane into detergent micelles. However, detergent can induce conformational change on transmembrane proteins. Our renewed detergent-free expression system can best preserve the native conformation of single-helix ATPase regulators for further structural studies.
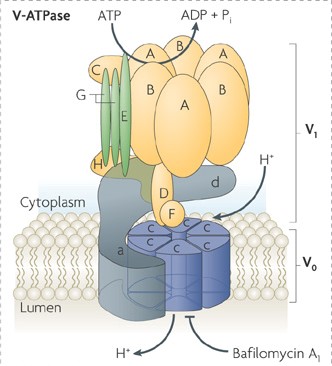
Figure 1. The structural model of ATPase regulators. (Nat. Rev. Mol. Cell Biol., 2010)
Creative Biostructure has exteinsive expertise in high quality ATPase regulators production using detergent-free membrane protein expression system, we can perform various strategies for Mempro™ detergent-free protein production, including:
-
Mempro™ single-helix ATPase regulators production using nanodiscs
Nanodiscs are the self-assembled system to stabilize membrane proteins removed from the membrane by membrane scaffold proteins (MSPs) in no need of detergent. Creative Biostructure can reconstitute single-helix ATPase regulators into nanodiscs in detergent-free process: Expressing the single-helix ATPase regulators in a cell-free system with the addition of pre-assembled nanodiscs.
-
Mempro™ single-helix ATPase regulators production using amphipols
The amphipathic amphipols have the ability to “trap” around transmembrane regions of proteins, allowing them to stay folded. Creative Biostructure can use A8-35 to solubilize single-helix ATPase regulators during purification step.
-
Mempro™ single-helix ATPase regulators production using poly (styrene-co-maleic acid) lipid particles (SMALPs)
The SMALPs are self-assembled by the simple addition of the SMA co-polymers. At neutral or alkaline pH, a disc-like structure assembles itself, encapsulating single-helix ATPase regulators in a form amenable to be purified.
These novel detergent-free approaches for single-helix ATPase regulators production can be obtained easily, and enabling more comprehensively structural and functional studies.
Creative Biostructure provides other various Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
C. Tribet, et al. (1996). Amphipols: Polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci U. S. A. 93: 15047-15050.
K. Geering (2005). Function of FXYD proteins, regulators of Na, K-ATPase. J. Bioenerg. Biomembr., 37(6): 387-92.
M. Jamshad, et al. (2011). Surfactant-free purification of membrane proteins with intact native membrane environment. Biochem. Soc. Trans., 39: 813-818.
R. Joseph, et al. (2010). Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol., 11: 50-61.