Mempro™ Membrane Anchor Helices of Peripheral Proteins Production Using Virus-Like Particles
Creative Biostructure now can provide custom Mempro™ membrane anchor helices of peripheral proteins production service using advanced virus-like particles (VLPs) platforms for various applications.
The membrane anchor helices of peripheral proteins are a large superfamily that contains the anchor regions of peripheral membrane proteins. Peripheral membrane proteins adhere to the membrane temporarily. They rely on the anchor region to achieve the attaching to the integral membrane proteins or penetrating the peripheral regions of the lipid bilayer. The anchor helices of peripheral proteins superfamily can be further catalogued into 10 families: Heat shock protein 9/12, Membrane anchor helix of BAR domain, Anchor helix of protein yopD, Membrane-binding domain of phosphotransferase IIA component, FATC domain, Anchor helix of glycosyl transferases group 1, N-terminal helix of Disabled-2, Lipid interacting helix of digalactosyldiacylglycerol synthase, N-terminal helix of Arf proteins and Lipid droplet anchoring peptide.
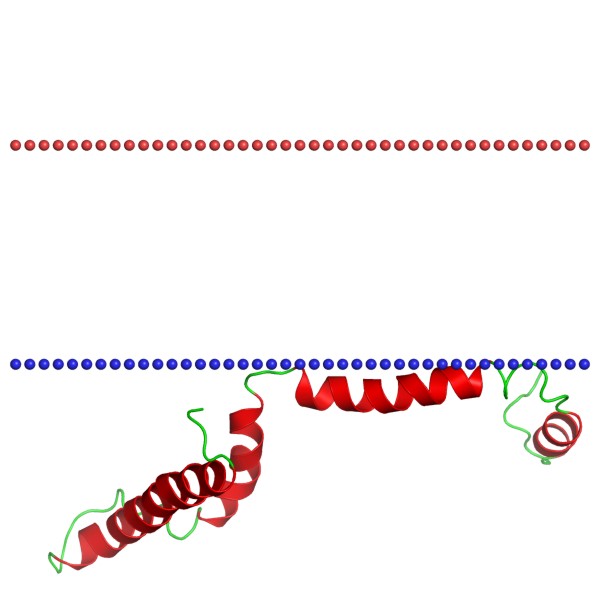
Figure 1. Structure of 12 kDa heat shock protein. (OPM)
With state-of-art VLPs platforms and years of experience in the field of recombinant membrane protein production, Creative Biostructure now can provide customers our most advanced custom Mempro™ membrane anchor helices of peripheral proteins production services with high quality. Our protein science team will provide the best strategy developing service according to the characteristics of target proteins or domains as well as your special requirements. Various VLPs production systems are available in Creative Biostructure for different applications, including bacteria, yeasts, green plants, mammalian cells and insect cells.
VLPs can be recognized as "empty viral protein shells", they are highly organized particles result in the self-assembly of viral structural proteins. VLPs can mimic the native virus in many ways. But all of them are non-infectious as they don't contain any viral DNAs or RNAs. VLPs have been developed as tools in various researches, such as vaccine developments and antigen deliveries. VLPs have been involved into the field of recombinant membrane protein production as an advanced method recently.
Creative Biostructure can achieve the production of target anchor helices of peripheral protein production using virus-like particles (VLPs) containing high concentrations of specific target anchor helices of peripheral proteins in their native conformation, called lipoparticles. We will perform the co-expression of target proteins and the viral structural core proteins within the chosen host cells. The expressed viral structural core proteins will achieve the self-assembly and then budding-off from the plasma membrane. The expressed target anchor helices of peripheral protein will be captured within the construction of specific lipoparticles at the same time. We can harvest the lipoparticles at the surface of the host cells with the target membrane protein being protected within the construction of lipoparticles. The entire process is free from detergents and mechanical disruptions.
Creative Biostructure can also provide other Mempro™ membrane protein production services with various advanced techniques. Welcome to contact us for more details.
Reference:
Sakamuro, D., Elliott, K. J., Wechsler-Reya, R., & Prendergast, G. C. (1996). BIN1 is a novel MYC–interacting protein with features of a tumour suppressor. Nature genetics, 14(1), 69-77.
Negorev, D., Riethman, H., Wechsler-Reya, R., Sakamuro, D., Prendergast, G. C., & Simon, D. (1996). The Bin1 Gene Localizes to Human Chromosome 2q14 by PCR Analysis of Somatic Cell Hybrids and Fluorescencein SituHybridization. Genomics, 33(2), 329-331.
A.Roldão, et al. Virus-like particles in vaccine development.Expert Rev. Vaccines, 2010,9(10): 1149-1176.
Feussner I, Wasternack C. The lipoxygenase pathway [J]. Annual review of plant biology, 2002, 53(1): 275-297.