Mempro™ PH Domain-Like Protein Production Using VLPs System
Creative Biostructure provides advanced custom Mempro™ pleckstrin homology (PH) domain-like protein production services in virus-like particles system. You can count on us all through your projects.
Virus-like particles (VLPs) are similar with virus, but are non-infectious owing to they do not have any viral genetic materials. VLPs are multisubunit self-assembly-competent protein structures (Figure 1), which are widely used in the field of vaccinology. VLPs derived from the Hepatitis B virus and composed of the small HBV derived surface antigen (HBsAg). Recently, virus-like particles carrying conformationally-complex membrane proteins (termed lipoparticles) have been applied for integral membrane protein production. Lipoparticles can incorporate a wide range of structurally intact membrane proteins, including G protein-coupled receptors (GPCRs), ion channels, and viral Envelopes. PH domains are the most common domains in the human proteome. The ability of PH domains is to bind phosphoinositides with high affinity and specificity, however, up to now, less than 10% of PH domain-like proteins share this property. With the further research, it is awared that PH domains can specifically bind phosphoinositides with high affinity are restricted to those phosphoinositides that possess a pair of adjacent phosphates in their inositol headgroup.
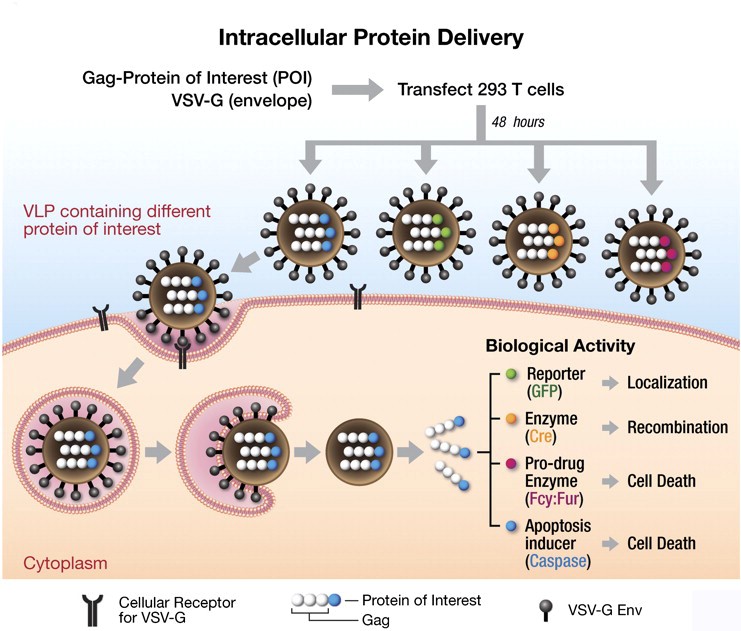 Figure 1. Application for virus-like particles system. (PNAS, 2011)
Figure 1. Application for virus-like particles system. (PNAS, 2011)
Up to now, virus-like particles can be performed for a wide range of applications, including:
- Antibody screening;
- Phage and yeast display;
- Vaccines production;
- Ligand binding assays;
- Other potential applications.
Creative Biostructure provides high-yield PH domain-like proteins in the stable, highly purified and native-conformation state. We can generate Lipoparticles from bacterial cells, yeast cells, insect cells, plant cells and mammalian cells for PH domain-like protein production. For instance, we can obtain lipoparticles from mammalian cells by co-expressing the retroviral structural core polyprotein, Gag, along with the membrane protein of interest. Gag core proteins self-assemble at the plasma membrane, where they bud off and capture target PH domain-like proteins. Since the PH domain-like proteins within lipoparticles are derived directly from the cell membrane surface without mechanical disruption or detergents, the native structure and orientation of PH domains are preserved.
Creative Biostructure can provide other various Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
D. P. Patterson, et al. (2012). Virus-like particle nanoreactors: programmed encapsulation of the thermostable CelB glycosidase inside the P22 capsid. Soft Matter, 8: 10158-10166.
M. A. Lemmon (2007). Pleckstrin homology (PH) domains and phosphoinositides. Biochem. Soc. Symp., 74: 81-93.
S. J. Kaczmarczyk, et al. (2011). Protein delivery using engineered virus-like particles. Proc. Natl. Acad. Sci. U. S. A., 108(41): 16998-17003.
S. Willis, et al. (2008). Virus-like particles as quantitative probes of membrane protein interactions. Biochemistry, 47(27): 6988-6890.