Mempro™ Plant-Based PLC-like Phosphodiesterases Production
Creative Biostructure has developed the comprehensive Mempro™ membrane protein production platform through years of experience, our seasoned scientists can provide high-quality phospholipase C (PLC)-like phosphodiesterases using plant-based expression system.
Creative Biostructure is highly-experienced in providing first-class membrane protein production services based on various types of plant hosts, including Nicotiana benthamiana (tobacco), Medicago rativa (alfalfa), Arabidopsis thaliana (A. thaliana), potato, maize, barley and lettuce. There are two different strategies for recombinant membrane protein production in plants: transient expression and stable transformation. Creative Biostructure would like to select either strategy producing membrane proteins to fulfill customers’ demands.
Mempro™ plant-based membrane protein production platform holds many advantages, such as animal-free, low protease activity, and low endotoxin. Animal-free recombinant membrane proteins are particularly crucial for customers concerned with experimental variables caused by trace animal components or mammalian pathogens. Recombinant membrane proteins expressed by our plant system are free of animal components, serum, endotoxins and antibiotics as well as human or animal infectious agents or other endogenous mammalian contamination. In addition, post-translational modification such as glycosylation and disulfide bonds can be introduced in plant system.
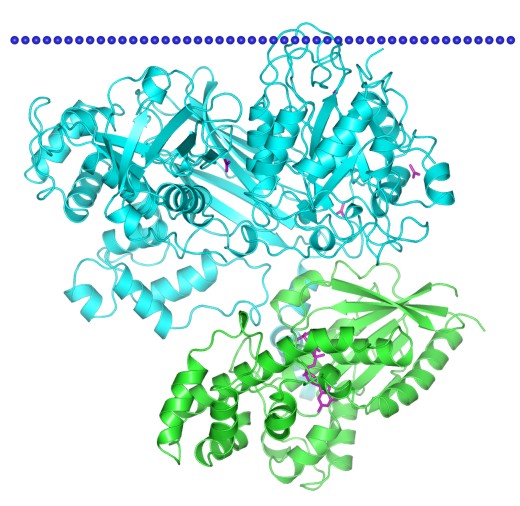
Figure 1. Structural model of Phospholipase C-beta isoform 3, complex with Galpha Q, structure 1. (OPM Database)
PLC-like phosphodiesterase possesses a structural domain consisting of a TIM beta/alpha-barrel, which can be found in several PLC like phosphodiesterases, including: mammalian phospholipase C isozyme D1, bacterial phosphatidylinositol-specific phospholipase C, and glycerophosphodiester phosphodiesterases, such as GlpQ from Escherichia coli and UgpQ from Agrobacterium tumefaciens. PLC isozymes can be activated by heterotrimeric G proteins and Ras-like GTPases to hydrolyze phosphatidylinositol 4,5-bisphosphate (PIP2) into the second messengers diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). PLC enzymes often play important roles in various signalling cascades. Base on the protein structure, PLC is divided into three types: β, γ and δ. In addition, The PLC catalyzing reaction is the major pass way for PIP2, which acts as an allosteric regulator or membrane anchor for various membrane proteins.
With the Mempro™ plant-based protein production platform, Creative Biostructure is able to express, isolate and purify PLC-like phosphodiesterases to facilitate the study of their biological functions. We can overcome the common difficulties encountered in cell-based expression system, such as misfolding, aggregation, inactivity, high endotoxin, poor stability and solubility, etc.
Creative Biostructure can also provide Mempro™ plant-based virus-like particles (VLPs) production services, Mempro™ animal-free recombinant protein production services and Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
A. Wiktorek-Smagur, et al. (2012). Green way of biomedicine – how to force plants to produce new important proteins. Transgenic Plants-Advances and Limitations, Chapter 3. doi: 10.5772/1409.
G. Kadamur, et al. (2013). Mammalian Phospholipase C. Annu. Rev. Physiol., 75:127-154.
PLC-like phosphodiesterase, TIM beta/alpha-barrel domain (IPR017946). (https://www.ebi.ac.uk/interpro/entry/IPR017946)