Protein Engineering and Analysis
Over the years, scientists from Creative Biostructure have been actively working on the characterization of proteins using a variety of techniques, as well as the engineering of proteins for various applications. The extensive experiences gained in these areas enable us to provide customized services in a timely and cost-effective manner.
Protein Engineering
Protein engineering is the process of modifying the structures of proteins and assigns them new and/or desirable properties in terms of activity, solubility, affinity, stability, specificity, resistance, etc. The most commonly applied approaches for protein engineering include de novo design, rational design, and directed evolution. Creative Biostructure offers protein evolution and engineering services covering the full cycle of protein engineering to satisfy customers’ requirements.
Protein evolution can be described as the changes over time in protein shape, composition, and structure. This process is closely related to the changes and selection of DNA polymorphisms and mutations due to protein sequence change responding to alteration in DNA sequence. Creative Biostructure adopts sensitive sequence profile methods and structure comparison methods to determine evolutionary relationships not detectable at the DNA-level.
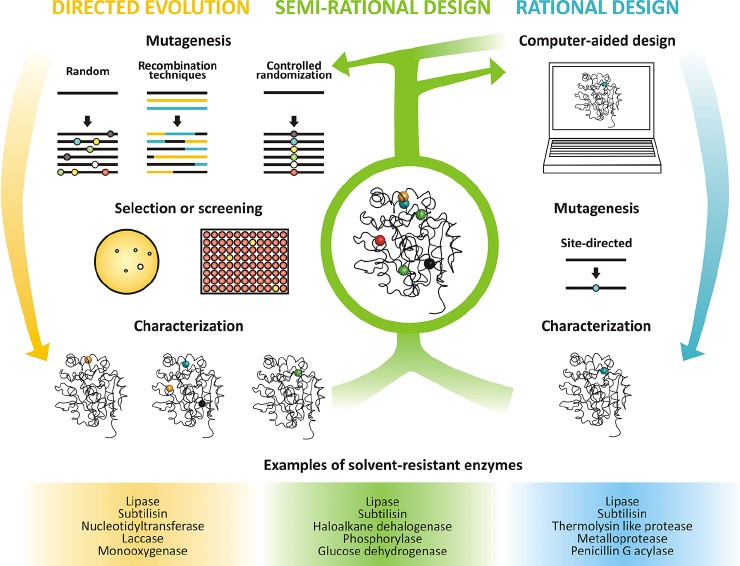 Figure 1. Strategies for protein
engineering
Figure 1. Strategies for protein
engineering
Protein Analysis
With the rapid growth of genomic sequencing data, an increasing number of proteins have emerged with great research interest. To further understand their biological roles and pave the way for structure-function relationship studies, many efforts have been made toward the development of various methods that have been proved effective in the biophysical characterization of biomolecules. Creative Biostructure provides a variety of sensitive techniques and appropriate strategies for protein analysis. The methods include but are not limited to epitope mapping, protein interaction analysis, and protein thermodynamics analysis.
Protein Engineering and Analysis Services at Creative Biostructure
Why Partner with Creative Biostructure?
- With a full understanding of various techniques and the requirements of every client, our scientists will select appropriate strategies to assist your protein analysis and/or protein engineering project.
- As for mutant library construction, we have strong expertise in de novo gene synthesis, which allows us to synthesize complex protein libraries without any dramatic increase in cost.
- With rational protein design, we make site-directed changes on the protein of interest based on structure-function relationship.
- Customizable solutions in a cost-saving and time-effective manner
Creative Biostructure also provides specialized mutant library services, including site-directed mutagenesis libraries, sequential permutation scanning libraries, and randomized and degenerated libraries. Please feel free to contact us for more information or a detailed quote.
Ordering Process
References
- Ryan TM, et al. (2013) Ammonium hydroxide treatment of Aβ produces an aggregate free solution suitable for biophysical and cell culture characterization. PeerJ. doi: 10.7717/peerj.73.
- Stepankova V. (2013) Strategies for Stabilization of Enzymes in Organic Solvents. ACS Catalysis 3(12):2823-2836.
- Kaumaya P. (2012) Protein Engineering: protein engineering methods and applications. ISBN 978-953-51-0037-9.