Mempro™ CRAL-TRIO Domain-Containing Protein Production Using Virus-Like Particles
Creative Biostructure can provide advanced custom Mempro™ CRAL-TRIO domain-containing protein production services based on virus-like particles system.
CRAL-TRIO domain is a structural domain that forms a hydrophobic binding pocket to bind small lipophilic ligands. The CRAL-TRIO domain contains several alpha helices as well as a beta sheet composed of 6 strands. Strands 2, 3, 4 and 5 form a parallel beta sheet with strands 1 and 6 being anti-parallel. CRAL-TRIO domain is found in GTPase-activating proteins (GAPs), a guanine nucleotide exchange factors (GEFs) and a family of hydrophobic ligand binding proteins, including the yeast SEC14 protein. CRAL binding protein carries 11-cis-retinol or 11-cis-retinaldehyde. While TRIO protein involved in coordinating actin remodeling, which is necessary for cell migration and growth.
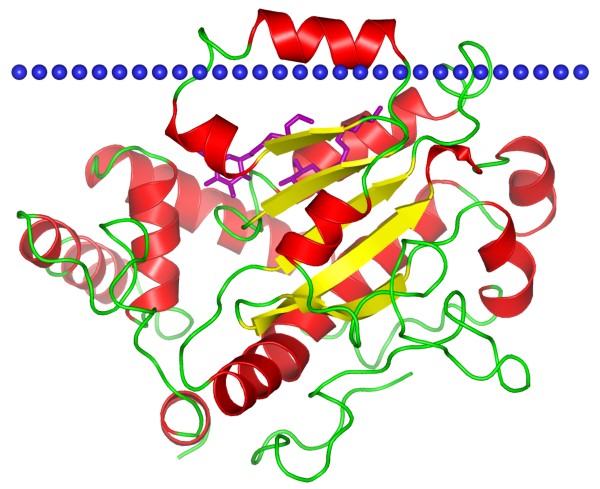
Figure 1. Phosphatidylinositol transfer protein sec14p. (OPM database)
Virus-like particles (VLPs) can simulate the native virus, but are non-infectious owing to they do not have any viral genetic materials. VLPs are self-assembly multiprotein structures, which are widely used in the field of vaccinology. VLPs derived from the Hepatitis B virus and composed of the small HBV derived surface antigen (HBsAg). Up to now, VLPs have been produced from components of a wide variety of virus families including bacteriophages (e.g. Qβ, AP205), Flaviviridae (e.g. Hepatitis C virus), Retroviridae (e.g. HIV), and Parvoviridae (e.g. adeno-associated virus). Recently, virus-like particles carrying conformationally-complex membrane proteins (termed lipoparticles) have been applied for integral membrane protein production. Lipoparticles can incorporate a wide range of structurally intact membrane proteins, including G protein-coupled receptors (GPCRs), ion channels, and viral Envelopes.
Creative Biostructure provides high-yield CRAL-TRIO domain-containing proteins in the stable, highly purified and native-conformation state. Lipoparticles can be produced from bacterial cells, yeast cells, insect cells, plant cells and mammalian cells for CRAL-TRIO domain-containing protein production. Well-characterized commercial Escherichia coli (E. coli) strains and insect cells are the most widely used systems for VLPs production. Mammalian cells are also widely used for VLPs production with the target to construct vaccine candidates and gene therapy agents. For instance, we can obtain lipoparticles from mammalian cells by co-expressing the retroviral structural core polyprotein, Gag, along with a desired membrane protein. Gag core proteins self-assemble at the plasma membrane, where they bud off and capture target membrane proteins. Since the CRAL-TRIO domain-containing proteins within lipoparticles are derived directly from the cell surface without mechanical disruption or detergents, the native structure and orientation of CRAL-TRIO domain-containing proteins are retained.
Creative Biostructure provides other various Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
A. Roldão, et al. (2010). Virus-like particles in vaccine development. Expert Rev. Vaccines, 9(10): 1149-1176.
D. P. Patterson, et al. (2012). Virus-like particle nanoreactors: programmed encapsulation of the thermostable CelB glycosidase inside the P22 capsid. Soft Matter, 8: 10158-10166.
J. M. bomar, et al. (2003). Mutations in a novel gene encoding a CRAL-TRIO domain cause human Cayman ataxia and ataxia/dystonia in the jittery mouse. Nature Gen., 35: 264-269.
S. Willis, et al. (2008). Virus-like particles as quantitative probes of membrane protein interactions. Biochemistry, 47(27): 6988-6890.