Mempro™ Detergent-Free CoA-Dependent Acyltransferases Production
The team of Creative Biostructure has excellent experts good at membrane protein production. Creative Biostructure enables to offer customized Mempro™ CoA-dependent acyltransferases production services using detergent-free expression system.
CoA-dependent acyltransferases is a superfamily which consists of 3 protein families: CAT-like family, Choline/Carnitine O-acyltransferase family and NRPS condensation domain family. The CAT-like family contains trimeric enzymes with the active sites seated between subunits. Choline/Carnitine O-acyltransferase family is the functional domain of multifunctional enzyme which has intersectant repeat of two CAT subunit-like domains. NRPS condensation domain family is monadelphous enzymes including tandem repeat of two Chloramphenicol acetyltransferase (CAT) subunit-like domains. CAT is one of the members of CAT-like family. This protein is a bacterial enzyme covalently links an acetyl group from acetyl-CoA to chloramphenicol, thus the combination of ribosomes and chloramphenicol is held back. A trimer of identical subunits forms the Chloramphenicol acetyltransferase, and the trimeric structure is firmed by several hydrogen bonds.
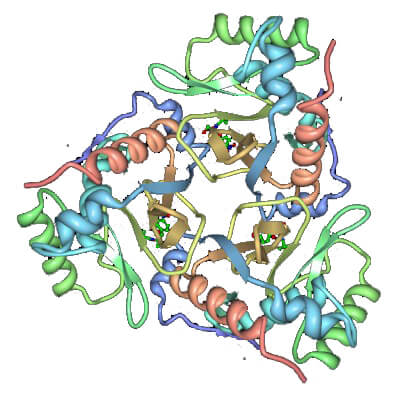
Figure 1. Ribbon diagram of the chloramphenicol acetyltransferase trimer with chloramphenicol bound. (Annu. Rev. Biophys. Biophys. Chem. 1991)
Creative Biostructure has rich professional experience in high quality CoA-dependent acyltransferases production using detergent-free membrane protein expression system, we can perform all kinds of strategies for Mempro™ detergent-free protein production, including:
- Mempro™ CoA-Dependent Acyltransferases Production Using Nanodiscs
Nanodiscs can be stabilized in solution through the assist of amphipathic helical scaffold protein, and they are being used to study isolation, purification and solubilization of membrane proteins. Nanodiscs are membrane imitations with commanded lipid composition which are stable and extremely soluble. The nanodiscs consist of a few lipids and two copies of MSP, which is named after membrane scaffold protein (MSP).
- Mempro™ CoA-Dependent Acyltransferases Production Using Amphipols
Amphipols (APols) meaning small amphipathic polymers which are enable to hold individual MPs water-soluble in their native state under the form of little hydrophilic complexes. Besides, amphipols can stabilize in aqueous solution with their inartificial nature state four well-authenticate integral membrane proteins.
- Mempro™ CoA-Dependent Acyltransferases Production Using Poly (styrene-co-maleic acid) Lipid Particles (SMALPs)
By using SMALPs, there is no need to collect proteins from membranes with possibly destabilising detergents, compared with nanodiscs, bicelles and amphipols, that is the most great advantage of this approach. It’s demonstrated that SMALPs may be a significant tool for the research of membrane protein structure and function.
These novel detergent-free approaches for CoA-dependent acyltransferases production can be obtained easily, and enabling more comprehensively structural and functional studies.
Creative Biostructure provides other various Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
Jamshad, M., Lin, Y.-P., Knowles, T. J., Parslow, R. A., Harris, C., Wheatley, M., . . . Overduin, M. (2011). Surfactant-free purification of membrane proteins with intact native membrane environment. Biochemical Society Transactions, 39(3), 813-818.
Rossmann, M., Schultz-Heienbrok, R., Behlke, J., Remmel, N., Alings, C., Sandhoff, K. Maier, (2008). Crystal structures of human saposins C and D: implications for lipid recognition and membrane interactions. Structure, 16(5), 809-817.
Ago, H., Kanaoka, Y., Irikura, D., Lam, B. K., Shimamura, T., Austen, K. F., & Miyano, M. (2007). Crystal structure of a human membrane protein involved in cysteinyl leukotriene biosynthesis. Nature, 448(7153), 609-612.
Gaczynska, M., Rock, K. L., Spies, T., & Goldberg, A. L. (1994). Peptidase activities of proteasomes are differentially regulated by the major histocompatibility complex-encoded genes for LMP2 and LMP7. Proceedings of the National Academy of Sciences, 91(20), 9213-9217.