Mempro™ DNase I-Like Protein Production Using Virus-Like Particles
Creative Biostructure now can provide custom Mempro™ DNase I-like protein production services using virus-like Particles for various applications.
DNase I-Like protein is a membrane protein superfamily that contains three families including endonuclease/exonuclease/phosphatase (EEP) family, arthropod phospholipase D and bacterial phosphatidylinositol-specific phospholipase C (OPM database). The EEP family is a structure domain that widely exist in many endonucleases and phosphatases involved in intracellular signaling. Members of arthropod phospholipase D belong to phospholipases and sphingomyelinases that are identified in a wide range of organisms, including bacteria, yeast, plants, animals, and viruses. The bacterial phosphatidylinositol-specific phospholipase C (PI-PLC) are small, water-soluble and calcium-independent enzymes that consist of a single domain folded as a (beta alpha) (8)-barrel. They interact with the membrane weakly and are involved in the cleaving of the natural membrane lipids PI, lyso-PI, and glycosyl-PI.
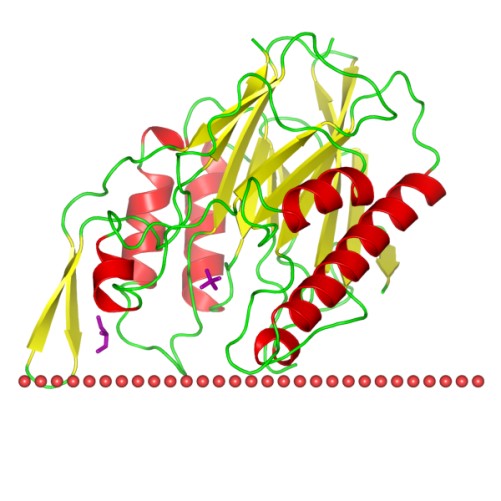 Figure 1. The schematic structure of Sphingomyelinase C (OPM Database)
Figure 1. The schematic structure of Sphingomyelinase C (OPM Database)
Creative Biostructure now can provide high-quality and high-yield Mempro™ custom DNase I-Like protein production services using virus-like particles (VLPs) with our most advanced VLPs technique and years of experience in membrane protein production. In Creative Biostructure, multiple VLPs production systems are available for customers to meet different experimental requirements, including bacteria, yeasts, green plants, insect cells and mammalian cells. With our service, your membrane protein production programs will become better and easier.
VLPs can be defined as "empty viral capsomere", they are highly organized particles that are result in the self-assembly of viral structural proteins. VLPs have been developed as powerful research tools for a quite long time. They are widely applied in vaccine developments, recombinant protein productions, heterologous epitope presentation programs and other kinds of researches. Many virus families have been used for the production of VLPs, including Parvoviridae, bacteriophages, Retroviridae, etc.
Creative Biostructure have developed unique lipoparticle techniques for the production of target DNase I-Like protein. Lipoparticles are VLPs that contain high concentration of specific membrane proteins within their construction. Our science team will perform the co-expression of chosen viral structural core particles (For example, the retroviral structural core polyprotein, Gag) along with the target DNase I-Like protein in the host cells. The recombinant viral structural core protein will proceed the self-assembly in the plasmid membrane of host cells to form VLPs, and then will bud-off from the plasmid membrane to construct the lipoparticles. The expressed target DNase I-Like protein will be captured into the construction of the lipoparticles at the same time. We are able to harvest the specific lipoparticles which contain the target protein without mechanical disruption or detergents, and push forward the program to next isolation and purification step. The native structure and orientation of target DNase I-Like protein will be protected perfectly by lipoparticles during the whole process.
Creative Biostructure also provides other various Mempro™ membrane protein production services. Welcome to contact us for more details.
Reference:
Pan C Q, Uumer J S, Herzka A, et al. Mutational analysis of human DNase I at the DNA binding interface: implications for DNA recognition, catalysis, and metal ion dependence[J]. Protein science, 1998, 7(3): 628-636.
Malicka-Błaszkiewicz M. DNase I-like activity and actin content in the liver of some vertebrates[J]. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 1986, 84(2): 207-209.
A.Roldão, et al. Virus-like particles in vaccine development.Expert Rev. Vaccines, 2010,9(10): 1149-1176.
Feussner I, Wasternack C. The lipoxygenase pathway [J]. Annual review of plant biology, 2002, 53(1): 275-297.