Mempro™ PX Domain-Containing Protein Production Using Virus-Like Particles
Creative Biostructure can provide advanced custom Mempro™ PX domain-containing protein production services in virus-like particles system.
Virus-like particles (VLPs) can simulate the native virus, but are non-infectious due to they do not have any viral genetic materials. VLPs are self-assembly multiprotein structures, which are widely used in the field of vaccinology. VLPs are derived from the Hepatitis B virus and consist of the small HBV derived surface antigen (HBsAg). It has been shown that virus-like particles carrying conformationally-complex membrane proteins (termed lipoparticles) can be applied for integral membrane protein production. Lipoparticles can incorporate a large variety of structurally intact membrane proteins, including G protein-coupled receptors (GPCRs), ion channels, and viral Envelopes. PX domain is a actual phospholipid-binding protein domain, which can bind preferentially with phosphatidylinositol 3-phosphate [PtdIns(3)P]. The structure of PX domain possesses an N-terminal, three-stranded β-sheet followed by a helical subunit made up of four α-helices. Proteins containing PX domains have been reported in a wide range of functions, including protein sorting, phospholipid metabolism and vesicular trafficking.
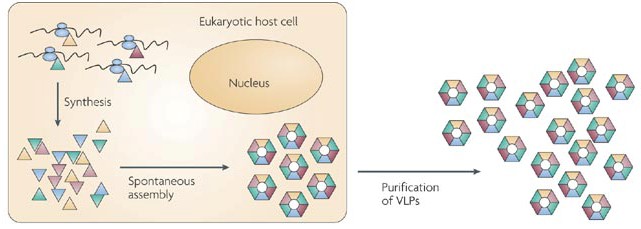 Figure 1. The formation of VLPs by the co-expression of virus structural proteins in the cytoplasm. (Nat. Rev. Microbiol., 2009)
Figure 1. The formation of VLPs by the co-expression of virus structural proteins in the cytoplasm. (Nat. Rev. Microbiol., 2009)
Virus-like particles can be performed for a wide variety of applications, including:
- Antibody screening;
- Phage and yeast display;
- Immunogens/vaccines production;
- Ligand binding assays;
- Nucleic acids and small molecules delivery.
- Other potential applications
Creative Biostructure offers high-yield PX domain-containing proteins in the stable, highly purified and native-conformation state. Lipoparticles can be produced from bacterial cells, yeast cells, insect cells, plant cells and mammalian cells for PX-domain protein production. Well-characterized commercial E. coli strains and insect cells are the most widely used systems for VLPs production. Mammalian cells are also widely used for VLPs production. For instance, we can obtain lipoparticles from mammalian cells by co-expressing the retroviral structural core polyprotein, Gag, along with a desired membrane protein. Gag core proteins self-assemble at the plasma membrane, where they bud off and capture target membrane proteins. Since the PX domain-containing proteins within lipoparticles are derived directly from the cell surface without mechanical disruption or detergents, the native structure and orientation of proteins are retained.
Creative Biostructure provides other various Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
A. Roldão, et al. (2010). Virus-like particles in vaccine development. Expert Rev. Vaccines, 9(10): 1149-1176.
L. F. Seet and W. Hong (2006). The Phox (PX) domain proteins and membrane traffic. Biochim. Biophy. Acta, 1761(8): 878-896.
P. Roy, et al. (2009). Prospects for improved bluetongue vaccines. Nat. Rev. Microbiol., 7: 120-128.
PX domain. (https://en.wikipedia.org/wiki/PX_domain)
S. Willis, et al. (2008). Virus-like particles as quantitative probes of membrane protein interactions. Biochemistry, 47(27): 6988-6890.
Virus-like particles. (https://en.wikipedia.org/wiki/Virus-like_particle#Assembly_of_VLPs).