For a full 10 years since returning to China in 2010, He Wanzhong, a researcher at the Beijing Institute of Biological Sciences, finally found a light in the darkness and handed in a satisfactory answer to himself and his supporters.
This light is that he led the team to originally developing a new type of visible electron microscopy(EM) labeling technology, which can directly synthesize gold nanoparticles in situ on the genetically encoded and expressed marker protein in the cell, thereby achieving accurate identification and positioning at the level of a single molecule on the ultrastructure of the cell, which provides a new tool for the study of the single-molecule level of the electron microscope ultrastructure of cells. Related research results were published on Nature Methods.
He Wanzhong called the past 10 years a “great adventure”. “Original exploration is not only inefficient, but also under pressure and risk. Some just insist on the belief that only genuine original work is done.”
Single-molecule visualization technology in cells at the ultrastructure level of EM is a long-awaited tool for cell biologists.
Nowadays, life science has entered the era of molecular biology, and people’s research on life phenomena has gone deep into the nano-scale level in single-cell and single-molecule. How to locate, identify, and manipulate target molecules within such a scale to obtain functional biochemical information will be of great significance to the interpretation of life phenomena.
He Wanzhong said, the recognition of protein molecules in cells has mainly relied on traditional antibody immunogold labeling technology, but it is affected by the stability and specificity of antibodies and antigens, chemical fixatives, and cell Influenced by factors such as slice permeability, colloidal gold particle size, etc., this technique usually has a low labeling efficiency (less than 10%), and even cannot label. In most cases, it can only label a very small part of the antigen on the surface of the cell slice, and it is also subject to background noise interference. In addition, traditional immunolabeling needs to label each slice, which is very expensive, time-consuming, and laborious for large-scale labeling of cell tissue samples.
The development of super-resolution fluorescence microscopy imaging technology has made cell imaging at the single-molecule level a reality. However, it can only image a small number of labeled molecules, and it is still “fuzzy” when imaging most unlabeled molecules and even organelles.
Therefore, cell biologists eagerly hope that the field of EM can also develop a green fluorescent protein (GFP) labeling technology that is widely used in the field of optical microscopy imaging, and realize the molecules and biological tissues on the ultrastructure samples of EM through genetic manipulation, identification, and precise positioning. Scientists call this technique “a visible electron microscopy labeling technique based on genetic code”.
To this end, scientists have tried to explore the development of EM-visible labels. For example, it is proposed to use heavy metal ions to directly combine with labeled proteins (including cysteine-rich metal-binding proteins, ferritin polymer, etc.) to form nano-metal particle tracers. Under the EM, the nanometal particles appear as a “big black spot”, similar to the GFP of fluorescence microscopy imaging, which allows the target molecule to “stand out” from the countless molecules in the cell.
In 2007, when He Wanzhong was a postdoctoral fellow at the California Institute of Technology in the United States, he invented the in-situ enlargement technology of frozen cell endocytosis of gold nanoparticles, which can trace the 1.4-nanometer gold clusters covalent cross-linked with each endocytosed antibody at the ultrastructure level, related results were published in Nature. However, this technology is based on the mechanism of endocytosis and is not suitable for labeling most molecules inside cells.
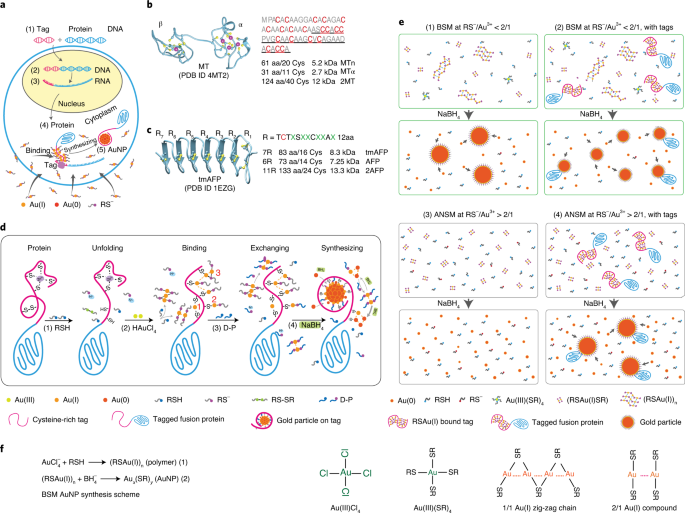
At this time, he keenly noticed that Dr. David DeRosier just proposed the development of a new concept of visible EM labeling based on cysteine-rich metalloprotein (MT), which has great potential to solve the above problems. Subsequently, He Wanzhong independently set up a laboratory, immediately began to transform MT into better MTn and MTa.
However, many technical obstacles have not been overcome, such as the inevitable “background noise” which leads to poor specificity, the inability of gold ions to enter cells effectively, the inability to resist chemical fixatives, the low synthesis efficiency of gold nanoparticles and the extremely small particles. No one has successfully developed a visible EM labeling technology at the single-molecule level of cell ultrastructure.
He Wanzhong was unwilling to stop there. “With the concept, it is a matter of solving this critical problem step by step. I believe it will definitely be resolved.”
On a weekend, a document he read mentioned that monovalent gold ions can have different binding strengths with nitrogen, oxygen, sulfur, and many other elements (the combination with sulfur is stronger than nitrogen and oxygen). He immediately thought of monovalent gold. There are also different bonding strengths between ions and different sulfhydryl-containing (sulfur-containing) compounds. This made his eyes bright: the 20 amino acids of protein are composed of a few elements such as carbon, hydrogen, oxygen, nitrogen, and sulfur. If he designs a method, find the sulfur (sulfhydryl) compound that binds to gold ions. As the reaction precursor, let the gold ion only combine with the sulfur of the cysteine on the label, and not combine with other elements, can it form specific gold nanoparticles while avoiding background noise?
After experiments, he has discovered that the concentration ratio of thiol anion to trivalent gold ion is ≥2:1, and another 2:1 soluble RSCu(I) gold thiolate can be formed, which cannot spontaneously form a nucleation center (this is the reason why gold particles were not formed in the control sample), He named this mechanism “self-nucleation inhibition mechanism (ANSM)”.
He explained that when a cysteine-rich labeled protein is added to the ANSM reaction system, the sulfhydryl group and gold ion on the labeled protein will form a chain polymer similar to RSCu(I) and become a nucleation center. There is no non-labeled self-nucleating chain polymer (background noise) in the solution, so it is not surprising that gold nanoparticles are specifically synthesized on the labeled protein after adding a strong reducing agent sodium borohydride solution.
Subsequently, He and the team spent another 7 years to overcome a series of technical challenges in the development and realization of the visible EM labeling technology.
They first successfully developed a series of techniques for specifically synthesizing 2-6 nanometer-sized gold particles for purified labeled proteins, and proved that gold nanoparticles are formed on a single labeled molecule. Using a simple E. coli system to optimize the experimental protocol for the specific synthesis of gold nanoparticles on the labeled protein in the cell, unprecedented high labeling efficiency was obtained.
Tests have proved that the program is highly mature and effective, with a high repetition rate, and can be widely used. “This also means that the problem of how to efficiently synthesize gold nanoparticles on labeled proteins under the premise of ensuring the ultrastructure of cells has finally been fully overcome.” He said.