NMR in association with multivariate chemometrics methodologies has been successfully employed in the quality control in industry. Quality control is particularly important in drug and pharmaceutical industry (Figure. 1). Pharmaceutical products have to be of high quality due to a number of reasons, such as identity of the active pharmaceutical ingredient (API) and the levels of impurities. 1H-NMR serves as a means to detect and quantify residual solvents such as methanol, DMF, petroleum ether and dioxane in products. In terms of extensive experience in quality control, Creative Biostructure has developed a set of NMR based screening tools for quality control in various industries such as food, beverage, drug and pharmaceuticals.
 Figure 1. Examples of applied NMR screening
Figure 1. Examples of applied NMR screening
Small quantities of compounds with a similar structure to the product molecule usually stand out in the NMR spectrum, which makes NMR a perfect way to detect side products, such as diastereomers. If there is more than one chiral center in a molecule, NMR can separate them. For instance, if two chiral centers exist in a molecule, there are four optical isomers: RR, RS, SR and SS, of which SR and SS are diastereomers, as a result, two sets of signals will appear in the NMR spectrum (Figure. 2). API in drugs usually has its specific structure, even a diastereomer may has significantly low activity, thus, detection of API by NMR has obvious advantages than other chromatographic approaches.
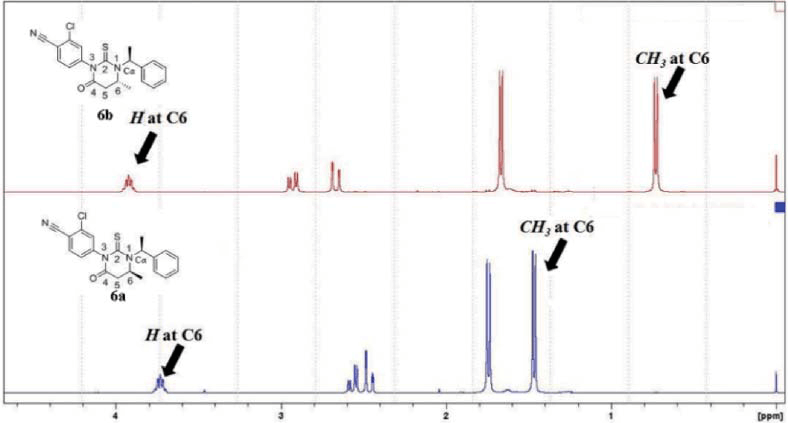 Figure 2. Expanded 1H NMR spectra of the diastereomers at C6a and C6b
Figure 2. Expanded 1H NMR spectra of the diastereomers at C6a and C6b
Organic solvents are used for production in pharmaceutical industry, and the leftover of these solvents are strictly examined for quality control. These organic solvents are routinely detected by NMR. Integration is used to measure the amount of impurity. The integral of the product is normalized to one per proton for comparison, the integral of the solvent may be different from the product, which indicates a molar ratio to the product. The sensitivity, as well as the resolution of NMR spectrometers, increases with the magnetic field strength of the super-conducting magnet, the resonance frequency of which, nowadays, ranges from 300 to 1000 MHz. Therefore, NMR screening may detect very small amount of solvent. The following table shows chemical shifts of several common solvents.
Table 1. Chemical shifts (ppm) of trace solvent.
| Trace solvent | In CDCl3 | In D2O | In DMSO-d6 |
| Acetic Acid | 2.10 | 2.08 | 1.91 |
| Acetone | 2.17 | 2.22 | 2.09 |
| Cyclohexance | 1.43 | 1.40 | |
| Dioxane | 3.71 | 3.75 | 3.57 |
| DMF | 2.88, 2.96, 8.02 | 2.85, 3.01, 7.92 | 2.73, 2.89, 7.95 |
| Ethanol | 1.25, 3.72 | 1.17, 3.65 | 1.06, 3.44 |
| Methanol | 3.49 | 3.34 | 3.16 |
| Pet. Ether | 0.88-1.26 | 0.89-1.25 |
Creative Biostructure could help with routine quality control, compound identification, quantitative analysis and assignment along with a wide variety of advance experiments. Please contact us for detailed inquiry.
Ordering Process
References
- Gottlieb H., Kotlyar V., and Nudelman A., (1997). NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 62: 7512-7515.
- Rundlof T., (2014). NMR spectroscopy: a superior tool for quality control of pharmaceutical products. European Pharmaceutical review. 5.
- Kumar V., Raghavaiah P., Mobin S., and Nair V., Diastereoselective syntheses of 3-aryl-5-(arylalkyl)-6-methyl-1-(1-phenylethyl) thioxotetrahydropyrimidin-4(1H)-ones: A stereochemical perspective from endo and exocyclic chiral centers. Organic & Biomolecular Chemistry.8: 4960-4970.

