Protein Thermodynamics Analysis
At Creative Biostructure we offer several thermodynamics analytical services to study the binding mechanism of your biomolecules. The approach we use for binding or formulation analysis is to measure and identify the most stable interaction or conformation of the protein using a number of biophysical approaches. Characterizing and stabilizing the conformation of a molecule is key to developing a successful, stable, and robust formulation.
Measurements of binding thermodynamics extends the characterization of biomolecular interactions, since binding affinity is a function of two quantities: the enthalpy (ΔH) and entropy (ΔS). By exploiting binding thermodynamics the potential of enthalpy and entropy correlations associated with chemical modifications in different regions of the lead molecule can be specified enabling a more robust and reliable drug discovery process.
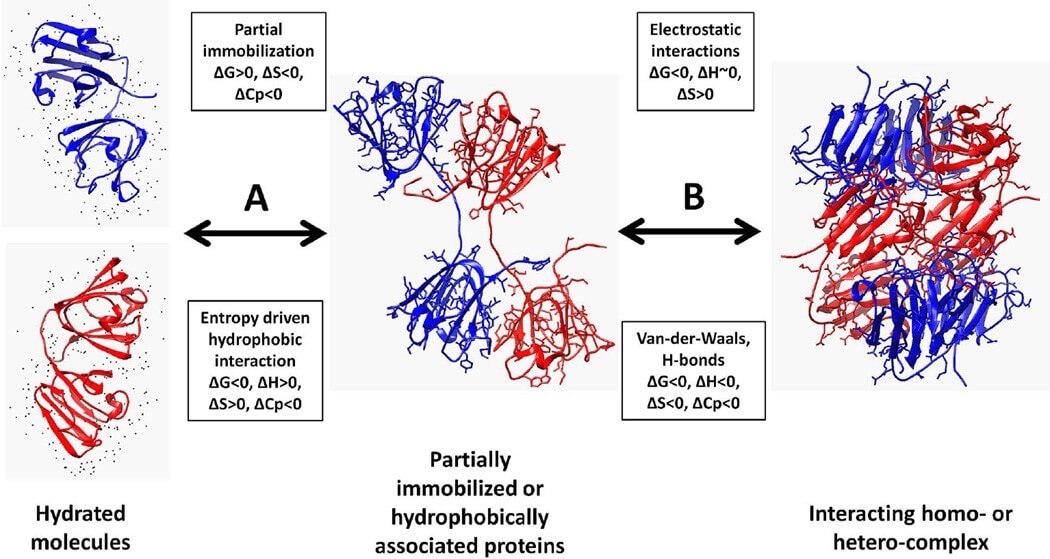 Figure 1. Thermodynamic view of protein association.
Figure 1. Thermodynamic view of protein association.
Creative Biostructure uses first-class instruments to analyze binding kinetics of a defined interaction between two biomolecules at a series of temperatures. Evaluation of interaction data obtained at varying temperatures provides van't Hoff and Eyring plots which yield thermodynamic constants for the equilibrium and transition state formation.
| Service category | Description |
| Protein thermal shift assay (PTSA) |
Measuring protein thermal stability; Various available approaches: reporter dyes, fluorescence wavelengths, FastPP, etc. |
| Differential Scanning Calorimetry (DSC) |
Monitoring heat effect by measuring the amount of energy that a sample absorbs or releases when it is heated or cooled; Applications including oxidative stability analysis, safety screening, melting behavior of complex organic materials determination, etc. |
| Thermal Gravimetric Analysis (TGA) |
Measuring against time or temperature; Samples composition determination and thermal stability prediction |
| Nano Differential Scanning Fluorimetry (NanoDSF) | Simple, fast and accurate analysis of protein folding and stability; Using the autofluorescence of tryptophan and tyrosine in proteins to detect their stability |
The facility provides real-time, label-free analysis for determination of thermodynamics of biomolecules, such as affinity, kinetics and concentration. Kinetic analysis including association and dissociation of biomolecules can be performed on up to 384 samples in one 384-well microplate.
Our services:
- High sensitivity detection
- Thermal stability measurements with temperature ramping experiment
- Automation friendliness allows multiple-plate assays in one run
- Biosensor re-racking allows maximum flexibility and operational cost savings
- Advanced software offers greater versatility in experimental design and data analysis
Please contact our application specialists for getting detailed information and an individual quote. We also provide various protein analysis services to support your project.
Ordering Process
References:
- Yuri V. Sergeev, et al. The thermodynamic analysis of weak protein interactions using sedimentation equilibrium. 2014. Curr Protoc Protein Sci. 77: 20.13.1–20.13.15. doi: 10.1002/0471140864.ps2013s77

