Antimicrobial resistance (AMR) is a major medical problem worldwide that affects human health and economic conditions. In a new study, Professor Ivan Dikic from the University of Frankfurt, Germany, together with his team found a new strategy to fight the bacteria. They revealed the mechanism of one Legionella and developed the first inhibitor. The relevant research was published online in Nature on May 23, 2018.
As drug resistance continues to spread, common infections such as pneumonia and salmonellosis are increasingly difficult to treat. There are two factors that contribute to this crisis of antimicrobial resistance: the negligence of human use of antibiotics and the failure to develop truly new antibiotics in more than 30 years. According to a report recently released by the World Bank, by 2050, antimicrobial resistance may reduce global gross domestic product (GDP) by 1.1% to 3.8%.
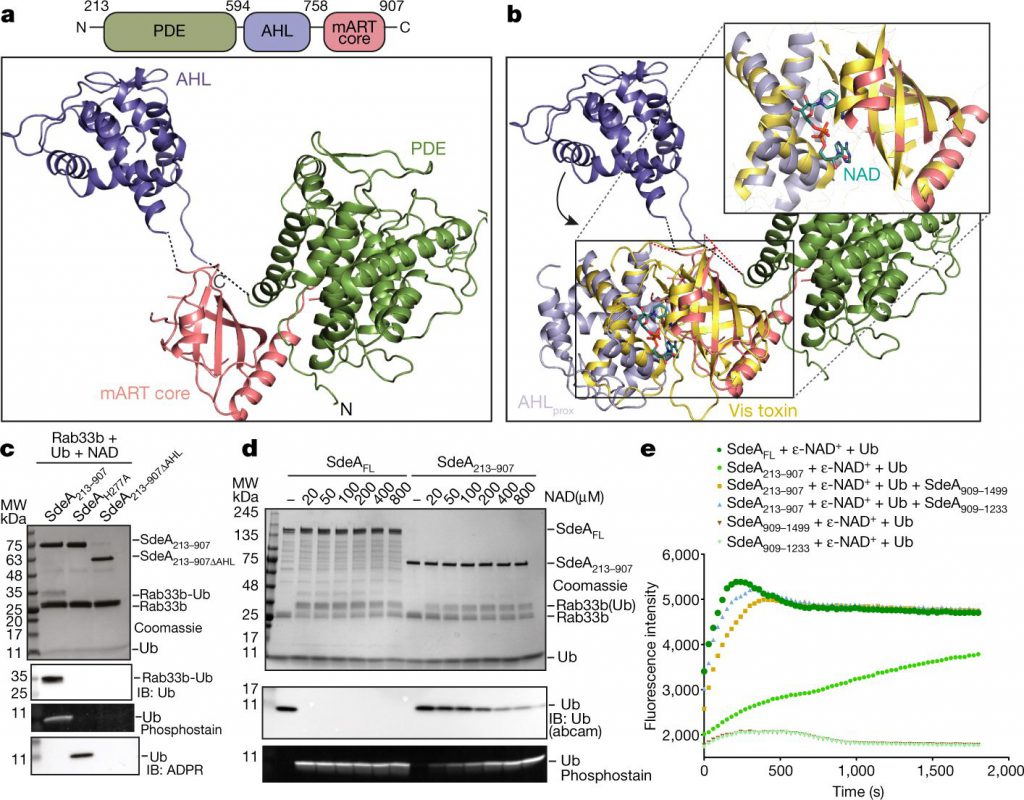
Scientific work is ongoing to better control microbial infections. One promising approach is to limit this damage by blocking microbial processes that damage the host cells and tissues. Dikic Laboratories has been conducting research in this area for the past decade. Dikic said, “We think we can find new treatments by using rationally designed drugs to target a specific class of bacterial effectors as a supplement to traditional antibiotics. In this way, pathogenic damage can be reduced, helping patients to better tolerate bacterial infection. This is a relatively new field and is attracting increasing attention in the scientific community.”
To prove that this strategy is a viable solution to bacterial infections, the Dikic team studied Legionella that is known to cause pneumonia and is particularly dangerous for patients with compromised immune function. Recently, these researchers participated in the identification of a new enzymology mechanism used by Legionella to control its host cells. Dr. Sagar Bhogaraju, the co-author of the dissertation and a member of the Dikic team, reported that “We have confirmed that the Legionella enzyme SdeA is a toxic bacterial effector. It promotes the spread of bacteria by targeting the ubiquitin system, of which the ubiquitin system is one of the powerful protective mechanisms of bacterial cells to resist stress.”
In this new study, the Dikic team reported that they had made further breakthroughs: they successfully resolved the atomic structure of SdeA. Dr. Sissy Kalayil, the paper’s lead author and member of the Dikic team, said, “This enzyme is quite unique and catalyzes the reaction through a two-step mechanism. Our results are very exciting because they reveal the atomic details of this mechanism and make it possible to rationally design inhibitors.”
The researchers also revealed how this bacterial effector is likely to select its target protein in the host cell and exert its effect by allowing ubiquitin to attach to these target proteins. They also developed the first inhibitor to block this reaction in vitro. Dikic commented, “Our basic findings allow us to confirm that the enzyme is drug-targetable. However, this is a preliminary finding. We still have a long way to go before we can use this new mechanism in therapy. We will certainly not stop there.” Most likely, Legionella is not the only bacterium that uses this mechanism.
Reference
Sissy Kalayil, Sagar Bhogaraju, Florian Bonn et al. Insights into catalysis and function of phosphoribosyl-linked serine ubiquitination. Nature, Published online:23 May 2018, doi:10.1038/s41586-018-0145-8