Herpesvirus is one of the most complex viruses both genetically and structurally. It spreads efficiently to host populations and leads to a range of human diseases, including congenital defects and cancers.
The herpesvirus assembly pathway produces three different types of capsids: A-, B-, and C-capsids. All three capsid types have a mature, angled shell and a similar assembly mechanism. However, little is known about the structure and assembly mechanism of the herpes simplex virus (HSV) capsid.
In a new study, Prof. Xiangxi, Wang, Prof. Zihe, RAO and Prof. Xinzheng, Zhang, together with the colleagues from Hunan Normal University and China Food and Drug Testing Institute reconstructed a B capsid structure of Herpes Simplex Virus Type 2 (HSV-2) with a resolution of 3.1 Å through Partition Reconstruction and Accurate Ewald Ball Correction method, deepening the understanding of HSV-2 B capsid assembly mechanism.
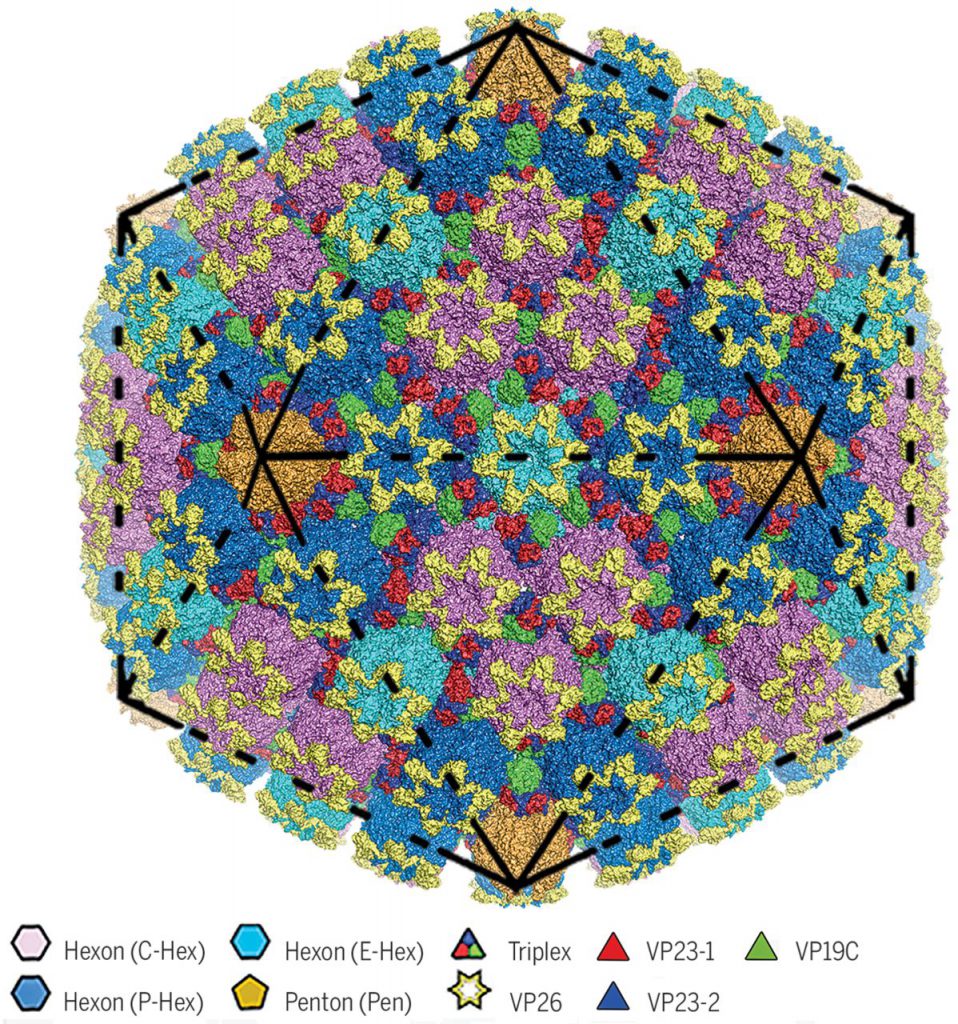
As one of the four major structural layers, a herpes virus capsid, approximately 125 nm in diameter, not only protects the viral genome from mechanical and other damage, but also releases the viral genome into the host cell nucleus during initial infection, and packages the viral genome during maturation.
The researchers found that there are four major conformers of the major capsid protein VP5. These conformers show significant differences in configuration and assembly patterns, resulting in a wide intermolecular network.
HSV-2 viral capsid assembly requires the orderly assembly of approximately 4000 protein subunits into hexamers, pentamers, and triplexes to assemble this viral capsid. Each trimer consists of two capsid proteins VP23 (the two VP23 proteins exhibit a significantly different conformation) and one capsid protein VP19C. These trimers are inserted between the hexamer and pentamer in a quasi-triplex position to bind HSV-2 viral capsids together. Six small-molecule capsid proteins, VP26, form a ring structure on the top of the hexamer, further stabilizing the HSV-2 viral capsid.
Based on this HSV-2 viral capsid structure, these investigators proposed a model for the ordered assembly of the HSV-2 viral capsid using the covalently linked lasso triangle formed by the trimer and its three VP5s. These basic assembly units are then clustered into high-order structures that conform to double symmetry, and guide the newly assembled intermediates to the correct T = 16 (referring to the number of triangles 16) geometry.
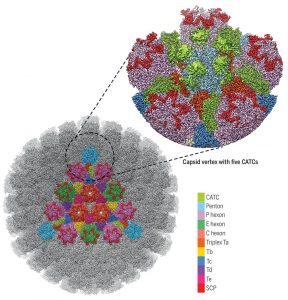
In another new study, Xinghong Dai and Z. Hong Zhou from the University of California, Los Angeles, used cryo-electron microscopy and obtained an atomic model of the HSV-1 capsid with CATC (capsid-associated tegument complex), comprising multiple conformers of the capsid proteins VP5, VP19c, VP23, and VP26 and tegument proteins pUL17, pUL25, and pUL36.
These findings help further understanding of the structural promoters of HSV viral capsid assembly and the structural basis of this viral capsid, which lay the foundation for the development of drugs that block HSV (whether HSV-1 or HSV-2) infections.
Reference
Shuai Yuan, Jialing Wang, Dongjie Zhu et al. Cryo-EM structure of a herpesvirus capsid at 3.1 Å. Science, 06 Apr 2018, 360(6384):eaao7283, doi:10.1126/science.aao7283
Xinghong Dai, Z. Hong Zhou. Structure of the herpes simplex virus 1 capsid with associated tegument protein complexes. Science, 06 Apr 2018, 360(6384):eaao7298, doi:10.1126/science.aao7298
Ekaterina E. Heldwein. Up close with herpesviruses. Science, 06 Apr 2018, 360(6384):34-35, doi:10.1126/science.aat3990