SARS-CoV-2 is a respiratory virus that causes COVID-19 disease. It attacks the human body through multiple steps. Entering cells deep in the lungs and hijacking the molecular machinery of human host cells to produce copies of the virus itself are the first two steps – both of these steps are essential for virus infection.
In a new study, researchers from the University of Arizona and the University of South Florida found that some existing compounds can simultaneously inhibit a key viral protein of SARS-CoV-2 required for replication in human cells – the main protease (Mpro) and cathepsin L, a human protein that is important for viruses to enter host cells, provide inspiration for the design of antiviral drugs against COVID-19. The relevant research results were recently published in the journal Science Advances, with the title of the paper “Structure and inhibition of the SARS-CoV-2 main protease reveals strategy for developing dual inhibitors against Mpro and cathepsin L”.
The co-corresponding author, Dr. Yu Chen, associate professor of molecular medicine at the University of South Florida Mossani School of Medicine, said, “If we can develop compounds that shut down or significantly reduce these two processes – virus entry and virus replication, this dual inhibition may enhance the effectiveness of these compounds in the treatment of coronavirus infections. To use an analogy, this is like killing two birds with one stone.” He is good at structure-based drug design.
This new research builds on previous research work – identifying and analyzing several promising existing antiviral drugs as drug candidates for the treatment of COVID-19. All selected drug candidates target Mpro to block the replication of SARS-CoV-2 in human cells grown in the laboratory.
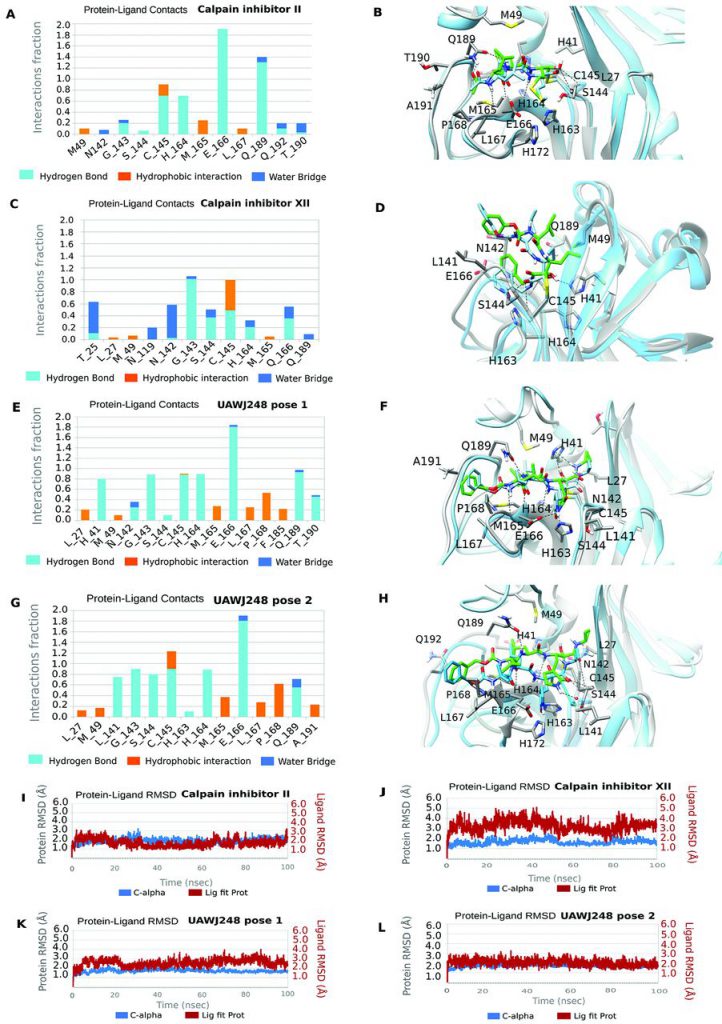
Two of these compounds, calpain inhibitors II and XII, are not as active as another drug candidate called GC-376 in biochemical tests. However, Michael Sacco, the first author of the paper and a doctoral student in Dr. Chen’s laboratory, said that calpain inhibitors, especially calpain inhibitor XII, actually kill SARS-CoV-2 in cell culture better than GC-376.
Sacco said, “We think that if these calpain inhibitors cannot inhibit the Mpro of this coronavirus so effectively, then they must be doing other things to explain their antiviral activity.” They learned from other research groups that calpain inhibitors can block other proteases, including cathepsin L, which is a key human host protease involved in mediating SARS-CoV-2 into cells.
In this new study, these researchers used advanced techniques, especially X-ray crystallography, to intuitively understand how calpain inhibitors II and XII interact with the viral protein Mpro. They observed that the calpain II inhibitor adhered to the target binding site as expected on the surface of SARS-CoV-2 Mpro. Unexpectedly, they also found that calpain XII inhibitors adopt a unique configuration called “inverted binding pose” to closely fit the active binding site of Mpro. (The tight fit optimizes the interaction between this inhibitor and the target viral protein, and reduces the activity of Mpro that assists the proliferation of SARS-CoV-2).
Dr. Chen said, “Our results provide us with useful structural information on how to design better inhibitors to target this key viral protein in the future.”
Dr. Chen said that in addition to simultaneously targeting the viral protease Mpro and human cathepsin L to improve potency (to obtain the desired drug effect at a lower dose), another benefit of dual inhibitors is that they have the potential to inhibit drug resistance.
SARS-CoV-2 will mutate, which may change its target gene sequence. These viral mutations deceive human cells, allowing the virus to attach to the cell surface membrane and introduce its genetic material into the cell, and can change the shape of viral proteins and their interaction with other molecules (including inhibitors) in the cell the way.
When SARS-CoV-2 is mutated in order to continue to proliferate, it may become resistant to certain inhibitors, thereby reducing the effectiveness of this compound. In other words, if the target gene sequence (lock) of this virus changes, then the key (inhibitor) is no longer suitable for that particular lock. But suppose the same key can open two locks to help prevent this virus infection; in this case, the two locks are Mpro (viral target protein) and cathepsin L (human target protein).
Dr. Chen said, “It is difficult for this virus to change two locks (two drug targets) at the same time. Therefore, dual inhibitors make antiviral drug resistance more difficult to develop. This is because even if the viral target protein changes, this type of compound is still effective against human host proteins that have not changed.”
These researchers continue to fine-tune existing antiviral drug candidates to improve their stability and performance and hope to apply what they have learned to help design new COVID-19 drugs. Their next research work will include solving how calpain inhibitors chemically and structurally interact with cathepsin L.
Reference
1.Michael Dominic Sacco et al. Structure and inhibition of the SARS-CoV-2 main protease reveals strategy for developing dual inhibitors against Mpro and cathepsin L. Science Advances, 2020, doi:10.1126/sciadv.abe0751.