X-ray Crystallography Services
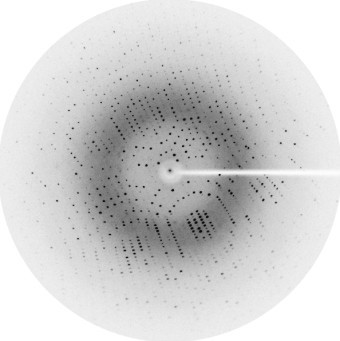
X-ray crystallography is currently one of the most favored methods for determining the structure of proteins and other macromolecules. The key requirement for a successful application of X-ray crystallography is to obtain single crystals of the target protein. Data is then collected by diffracting X-rays from the single crystal, which exhibits an ordered pattern of atomic orientation. The arrangement of atoms and molecules in the crystal can be deduced from the measurement of X-ray scattering. For a considerable period, protein structures analyzed through X-ray crystallography experiments and deposited in the Protein Data Bank accounted for nearly 90% of the resolved structures. This highlights the widespread utilization of X-ray crystallography in structure determination.
Creative Biostructure offers three types of protein X-ray crystallography services. We provide X-ray crystallography services in our state-of-the-art crystallography equipment and has developed an X-ray crystallography pipeline that encompasses all technical stages, from gene synthesis to structure determination. You can also explore our fast-track crystallization service for selected protein targets. Our experienced crystallographers collaborate closely with customers to ensure a rapid turnaround and deliver reliable results.
Gene-to-structure Services
- Plasmid construction: full-length, site-mutated, truncated, appropriate tag
- Protein expression system: bacterial system, yeast system, insect cell/baculovirus system, mammalian cell system, plant system, cell-free protein production
Custom X-ray Crystallography Services
Our services are accompanied by well-defined methodologies and instruments, yet they provide a great deal of flexibility based on the customer's requirements. We can either prepare crystallization-grade proteins from scratch or obtain them from customers.
- Initial crystallization screening only (using high-throughput crystallization analyzer)
- Crystal optimization only
- Data collection only (utilizing either Synchrotron or X-ray spectrometer)
- Structure determination only (model building, refinement, and quality verification)
Fast-track Crystallization Services
Creative Biostructure has multiple ready-made high-purity proteins and crystal structures developed internally that can be used for co-crystallization and compound soaking.
- Over 100 relevant targets ready for protein-ligand structure determination
- Saving time with pre-defined conditions for protein production, purification, and crystallization
AI model-assisted determination of protein crystal structures improves their accuracy, especially in complex or ambiguous regions, and accelerates the structure analysis process.
Our Featured Crystallization Solutions
Creative Biostructure can provide various crystallization strategies, particularly for membrane protein crystallization. The detailed services are summarized as follows.
| Featured Crystallography Services | Feature |
|---|---|
| Fusion Protein-Assisted Crystallization | Leverages fusion partners like maltose-binding protein to enhance the solubility, stability, and crystallization potential of target proteins, facilitating structural biology studies. |
| Co-crystallization | Co-crystallization retains the unique crystalline structures with their multiple components (e.g., proteins, DNA/RNA, chemical compounds, metal ions). |
| Eutectic Freeze Crystallization | Takes advantage of the difference in freezing points between different compounds in solution and achieves separation and purification by cooling the whole system and precipitating substances in a different order according to different temperature gradients. |
| Crystalline Sponge Method | Makes the crystallization step in it unnecessary and promises to revolutionize the determination of molecular structure. |
| Bicelle-Protein Crystallization | Providing a more bilayer-like environment for membrane proteins than in detergent micelles, enabling the use of standard crystallization screening methodology for membrane proteins. |
| Lipidic Cubic Phase (LCP) Crystallization | LCP has a membrane-mimetic matrix suitable for stabilization and crystallization of membrane proteins in lipidic environment. |
| Controlled In Meso Phase (CIMP) Crystallization | CIMP stabilizes membrane proteins in meso phase and allows for direct monitoring of phase transformation and crystallization events. |
| Trace Fluorescent Labeling Crystallization | Great for the detection and identification of crystals by covalently labeling a fluorescent probe on the protein. |
| Crystallization Chaperone Strategies | Co-crystallization of challenging membrane protein targets with soluble protein chaperones. |
| Crystallization with Mutant Library Approaches | Improvement of protein solubility and crystallization assisted by mutant library construction and screening. |
Your Specific Sample Types for Crystallization
Technology Platforms
Our platform can support a continuous pipeline encompassing all stages involved in the structure determination of peptides, proteins, and other macromolecules, including their complexes with small molecules and nucleic acids.
X-ray Crystallography Platform
This technology platform relies on high-throughput screening technologies for the expression, purification, and structural characterization of difficult membrane proteins, such as GPCRs, ion channels, transporters, and GPI-anchored proteins.
Membrane Protein Platform
Key Advantages
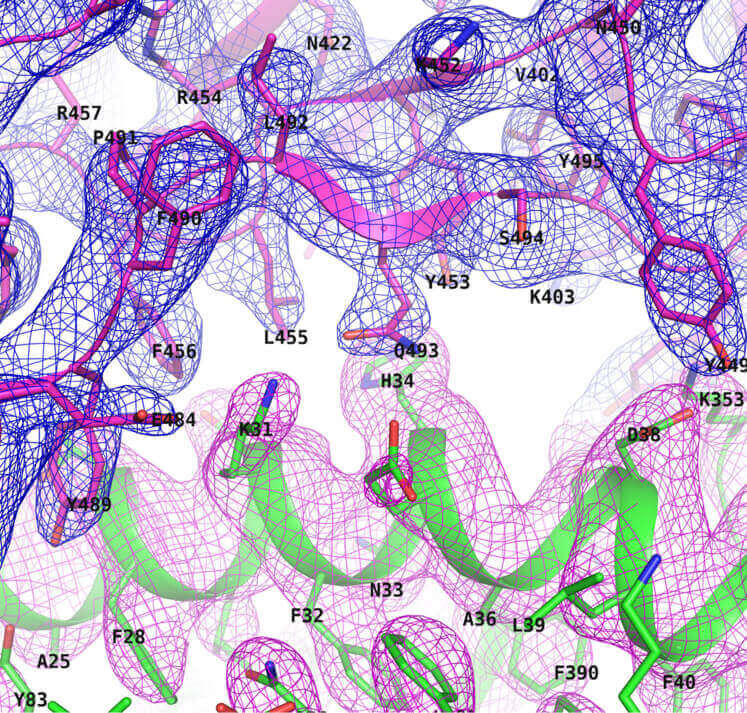
- To obtain a three-dimensional structure of a protein/protein-protein complex/protein-small molecule complex with high atomic resolution, and to provide structural information useful for understanding protein function and accelerating the process of rational drug design.
- It is not limited by the molecular weight of the sample.
- It helps reduce the technical barrier of obtaining single crystals of specific proteins, such as membrane proteins, that are difficult to process by conventional methods.
- The algorithm for protein structure analysis is well-developed, and the established model has high confidence.
Resources
Frequently Asked Questions
-
Difference between protein structure determined by cryo-EM and X-ray crystallography?
Cryo-EM is best suited for studying flexible or heterogeneous protein complexes, while X-ray crystallography is best suited for studying highly ordered, crystalline proteins.
-
How long does it take to receive X-ray crystallography results?
The turnaround time for X-ray crystallography results can vary based on the complexity of the project and the specific requirements. Typically, we strive to deliver results promptly without compromising the quality of the analysis.
-
How do you ensure the quality of X-ray crystallography data in your services?
Our X-ray crystallography services prioritize quality and reproducibility. We adhere to rigorous crystallographic standards, implement automated data collection techniques, and perform extensive validation procedures. This ensures that the crystallographic data obtained is of high quality and can be reliably reproduced.
Ordering Process
References
- D'Arcy A, et al. Microseed matrix screening for optimization in protein crystallization: what have we learned?. Acta Crystallographica Section F: Structural Biology Communications. 2014, 70(9): 1117-1126.
- Hediger M R, et al. BioFET-SIM web interface: Implementation and two applications. PloS One. 2012, 7(10): e45379.