Mempro™ Detergent-Free Lipocalin Production
Creative Biostructure can provide unmatched custom Mempro™ lipocalin production services using detergent-free expression system.
Lipocalins are well known as the extracellular proteins that can transport small hydrophobic molecules. Members of the lipocalin family share a common tertiary structure architecture, which is an eight stranded antiparallel β-barrel with a repeated topology containing an internal ligand binding site. Many lipocalins have been reported to bind to specific receptors such as retinol (Vitamin A) binding protein (RBP), alpha1-microglobulin, major urinary protein (MUP) and odorant-binding protein (OBP). Lipocalins play important roles in biological processes, including cancer cell interactions, immune response, prostaglandin synthesis, and pheromone transport. Creative Biostructure can provide the best production of C2 domian-containing proteins to facilitate the structural and functional studies.
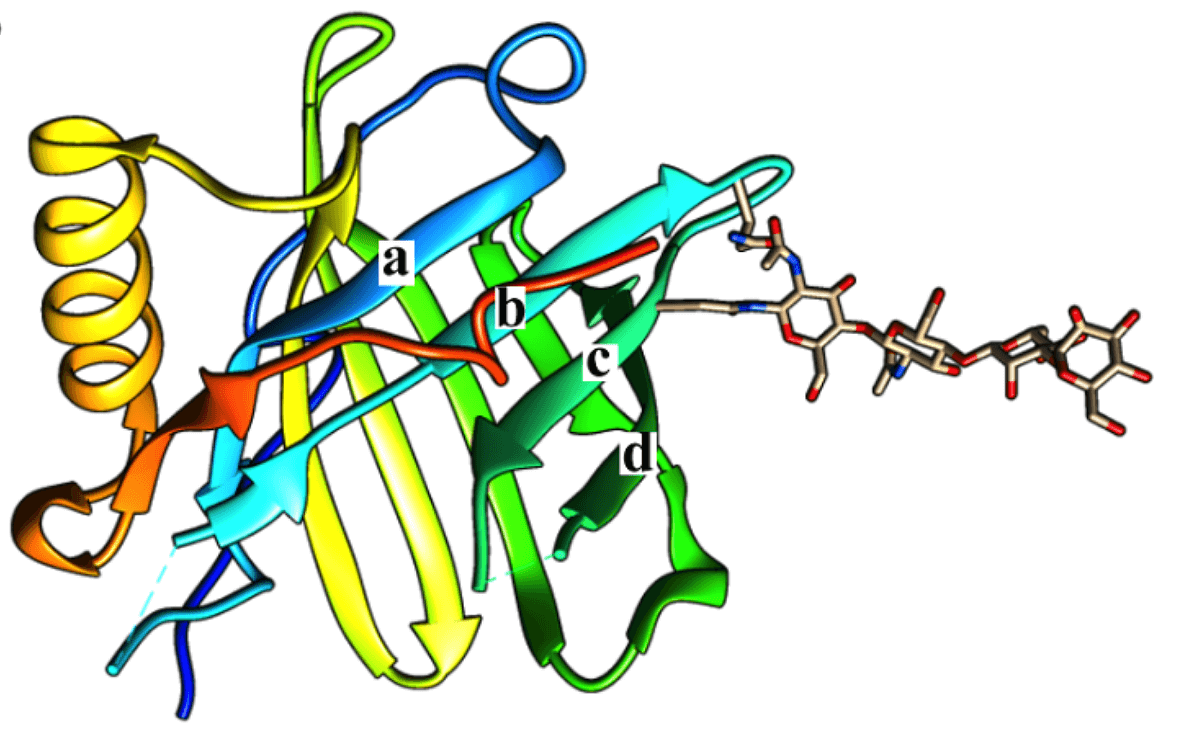 Figure 1. Three-dimensional structures of lipocalin coupled with an N-linked glycan. a, b, c, and d indicate the directions of the β-strands. (Toxins, 2016)
Figure 1. Three-dimensional structures of lipocalin coupled with an N-linked glycan. a, b, c, and d indicate the directions of the β-strands. (Toxins, 2016)
Creative Biostructure has great expertise in high-yield lipocalin production using detergent-free membrane protein expression system, we can perform various strategies for Mempro™ detergent-free membrane protein production, including:
- Mempro™ lipocalin production using nanodiscs
Nanodiscs are the self-assembly nanotechnology to stabilize integral membrane proteins removed from the membrane by membrane scaffold proteins (MSPs) in no need of detergent. Creative Biostructure can provide combination of lipocalins expressed by cell-free expression system into pre-assembly nanodiscs during purification process.
- Mempro™ lipocalin production using amphipols
The amphipathic amphipols have the ability to “trap” around membrane proteins, allowing them to stay folded. Creative Biostructure can provide A8-35 to solubilize lipocalins.
- Mempro™ lipocalin production using poly (styrene-co-maleic acid) lipid particles (SMALPs)
The SMALPs are self-assembled by the simple addition of the SMA co-polymers. At neutral or alkaline pH, a disc-like structure assembles itself, encapsulating lipocalins in a form amenable to be purified.
These novel detergent-free purification approaches for lipocalin production can be obtained easily, and facilitate more comprehensively structural and functional analysis.
Creative Biostructure can provide various custom Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
A. Schiefner and A. Skerra (2015). The menagerie of human lipocalins: A natural protein scaffold for molecular recognition of physiological compounds.Acc. Chem., 48(4): 976–985.
C. Tribet, et al. (1996). Amphipols: Polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci U. S. A. 93: 15047-15050.
D. R. Flower (1996). The lipocalin protein family: structure and function. Biochem. J., 318(Pt 1): 1-14.
Lipocalin. (https://en.wikipedia.org/wiki/Lipocalin)
M. J. Hernández-Vargas, et al. (2016). An insight into the triabin protein family of american hematophagous reduviids: functional, structural and phylogenetic analysis. Toxins, 8(2): 44.
M. Jamshad, et al. (2011). Surfactant-free purification of membrane proteins with intact native membrane environment. Biochem. Soc. Trans., 39: 813-818.
R.O. Ryan (2010). Nanobiotechnology applications of reconstituted high density lipoprotein. J. Nanobiotechnol., 8(1):28-37.