Epitope Mapping Using Crystallography
With skills and knowledge on co-crystal structures, Creative Biostructure provides epitope mapping services specializing in X-ray crystallography technique. Epitope mapping aims at identifying the binding sites of antibodies on their target antigens. It has become increasingly vital in discovery and development of new vaccines and antibody drugs. Epitope identification and characterization of an antibody will greatly facilitate drug discovery and design.
Epitopes can be classified into two main groups: linear and conformational epitopes. A linear epitope is the continuous section of the sequence; a conformational epitope, on the other hand, is composed of discontinuous subsections of an antigen sequence. Previous studies about epitopes show that most of the antigen-antibody interactions depend on conformational epitopes.
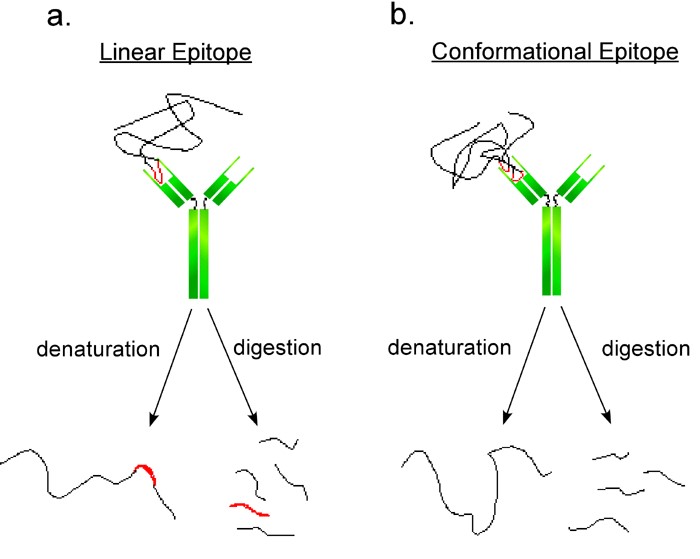 Figure 1. Schematic representation of two antibodies interacting with linear and conformational epitopes.
Figure 1. Schematic representation of two antibodies interacting with linear and conformational epitopes.
(a. Linear epitopes are short and continuous. After denaturation the linear epitopes may still be able to bind the antibody. b. Conformational epitopes are domains of proteins composed of specific regions of protein chains. After denaturation the discontinuous epitope can no longer bind the antibody.)
Epitope mapping is the process of identifying and characterizing the minimum molecular structures recognized by immune system, mainly T and B cells. Most epitopes recognized by antibodies or B cells are three-dimensional surface features of a protein antigen; these features fit the complementarity determining regions (CDRs) precisely and thus are bound by antibodies. Understanding this structure recognition mechanism relies on determining the structure of the antibody-antigen complex. Once the structure is known, physical interactions between the antigen and antibody can be confirmed and depicted at the atomic level. Therefore, X-ray crystallography is considered to be the most unambiguous method for epitope mapping.
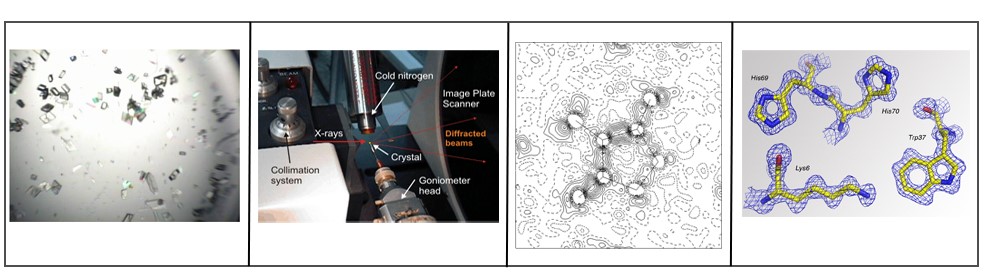 Figure 2. Epitope mapping using X-ray crystallography method
Figure 2. Epitope mapping using X-ray crystallography method
Based on high standard X-ray crystallography methods, Creative Biostructure offers comprehensive epitope mapping services for both conformational and linear epitopes with high accuracy. In addition, our protein analysis and high-throughput crystallization services will greatly facilitate the epitope mapping project as well. We have extensive experience in crystallizing antibody-antigen complexes and resolving their 3-D structures. In particular, we are able to map the epitopes of a large variety of antibody forms, including intact IgG, Fab, scFv, F(ab')2, diabody, minibody, minoantibody, tandem scFv and nanobody and affibody.
Our services:
Overlapping peptide library construction
Binding activity test by ELISA/WB
X-ray crystallography
3D-EM (Electron Microscope)
LC-MS
Features:
-
Broadest applicability
Linear, conformational, discontinuous epitopes and complex epitopes involving multimeric protein complexes
-
Re-usable arrays for multiple screenings
Comparative mapping and epitope fingerprinting of up to 100’s of samples
-
Highest success rate
Unmatched sensitivity through high peptide density
- Structurally and functionally customized arrays
Including PTM’s, cyclizations, helices, β-sheets, etc.
Please contact us for more details.
Ordering Process
References:
Gershoni et al. 2007. Epitope Mapping. BioDrugs. 21 (3): 145–56. doi:10.2165/00063030-200721030-00002.

