Custom Hydrophobic Interaction Chromatography Service
Creative Biostructure provides custom hydrophobic interaction chromatography service for separation and purification of biological macromolecules.
Hydrophobic Interaction Chromatography (HIC) is a special chromatographic method that performs the separation and purification of biological macromolecules by the difference of their hydrophobicity, which is the same as reversed phase chromatography (RPC). HIC is widely performed as a useful complement to other purification methods. It is an ideal next step method for samples that have been separated by ion-exchange chromatography (IEX) or subjected to ammonium sulfate precipitation, which is usually performed for initial sample concentration and clean-up. The sample contains a high salt concentration in both of the two situations, which means the sample can be applied directly to the HIC medium without or with just little preparation. HIC can be used for any step in a purification strategy including the capture step, the intermediate step and the polishing step.
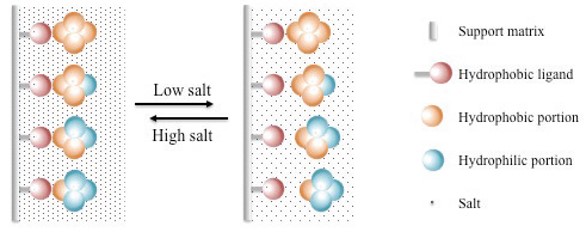 Figure 1. Schematic illustration of hydrophobic interaction chromatography.
Figure 1. Schematic illustration of hydrophobic interaction chromatography.
HIC separated the sample components according to their different surface hydrophobicity by reversible interactions between biological macromolecules and the hydrophobic surfaces of HIC mediums. Unlike other chromatographic methods such as IEX and AC, the reversible interaction between target molecules and HIC mediums were enhanced by high salt concentration, and lowering the salt concentration in the running buffer weakens the HIC interaction. At the conditions with high salt concentration, hydrophobic biological macromolecules interact with the HIC medium. As the ionic strength is reduced, interaction is reversed and the components are eluted. Component with the lowest degree of hydrophobicity is eluted first and the most hydrophobic molecule eluted last as it requires the greatest reduction in ion strength to reverse the hydrophobic interaction.
Creative Biostructure now provides custom hydrophobic interaction chromatography for advanced biological macromolecule separation and purification. Different experimental systems from both AKTA and Bio-Rad are available. We also provide multiple kinds of HIC medium to our customers to meet different requirements. With years of experience, our science team will take the structure of target molecules and reversible hydrophobic interactions into consideration. By doing this, we will establish the optimal HIC strategy for each individual case to meet your different requirements.
We can also provide other chromatography and purification services including but not limited to:
Affinity Chromatography
Ion-exchange Chromatography
Size-Exclusion Chromatography
Reversed Phase Chromatography
TFF/Diafiltration
Please feel free to contact us to discuss your project!
Ordering process
References:
- Kennedy, Robert M. "Hydrophobic‐interaction chromatography." Current Protocols in Protein Science (1995): 8-4.
- Geng, Xindu, and Xiaoqing Chang. "High-performance hydrophobic interaction chromatography as a tool for protein refolding." Journal of Chromatography A 599.1-2 (1992): 185-194.
- O’Farrell, Paul. "Hydrophobic Interaction Chromatography." Protein Purification Protocols (1996): 151-155.

