Bicelle-Protein Crystallization
The structure determination of membrane proteins remains a big challenge due to the intrinsic difficulty in expressing, purifying and crystallization of membrane protein targets. Crystallization trials for membrane proteins are tedious and conventional methods usually require extensive detergent screening to identify an optimal condition that is not just able to dissolve and stabilize membrane proteins, but also compatible with crystallization media.
Creative Biostructure has been actively developing and optimizing crystallization methods for membrane proteins. One great strategy to tackle difficulties in membrane protein crystallization is our bicelle-protein crystallization technique. Bicelles are small bilayer disks formed in lipid/amphiphile mixtures, into which membrane proteins can be incorporated. At low temperature, bicelle-forming lipid/amphiphile mixtures are not viscous, but they tend to develop a gel-like consistency at higher temperatures. Thus, with bicelle forming lipid/amphiphile mixtures it is possible to access a variety of lipid bilayer structures simply by varying temperature.
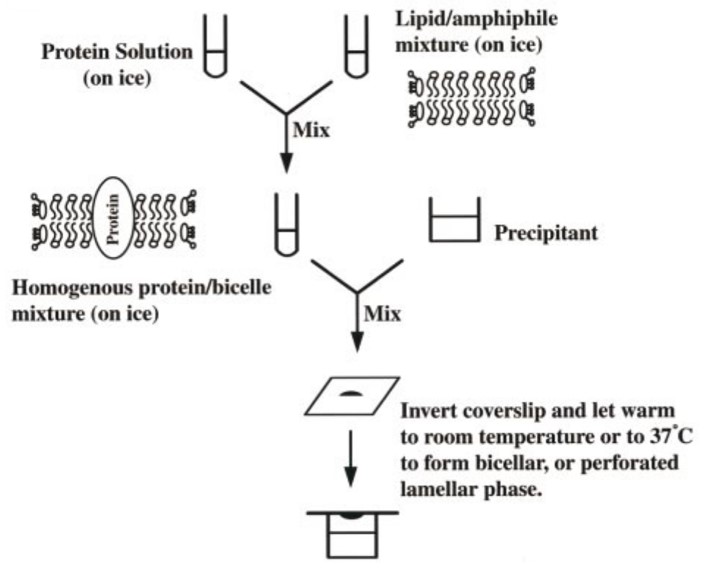 Figure 1. Bicelle crystallization method
Figure 1. Bicelle crystallization method
The bicelle crystallization method is outlined in Figure 1. First, the protein is mixed with bicelle components and kept on ice where the mixture remains liquid. Then, for each trial, the protein/bicelle mixture is combined with a precipitant solution on a silanized coverslip or in the well of a sitting drop apparatus. The components can be easily mixed by simply pipetting up and down. The drop is then sealed over a reservoir of precipitant solution and if the gel-like phase is desired, the set of trials can be moved to an incubator at 37°C.
Our bicelle-protein crystallization technique provides a more bilayer-like environment for membrane proteins than in detergent micelles, and the temperature-sensitive property of bicelles enables the use of standard crystallization screening methodology for membrane proteins. There is no need for the protein to escape to a lamellar-like phase for crystal formation as in lipid cubic phase crystallization. Our extensive optimization also increases success rate by favoring bicelle crystallization and avoiding conditions producing false leads.
Please feel free to contact us for a detailed quote.
Ordering Process
References
Faham S, Bowie JU. (2002) “Bicelle crystallization: a new method for crystallizing membrane proteins yields a monomeric bacteriorhodopsin structure”. J Mol Biol 316(1):1-6.

