High-Pressure NMR Service
The combination of high-resolution NMR spectroscopy with pressure perturbation, called variable pressure NMR spectroscopy or high-pressure NMR spectroscopy, is a rapidly developing and promising technique for the future. The importance of this method lies in its ability to systematically detect and analyze the structure and thermodynamic stability of high-energy sub-states in proteins.
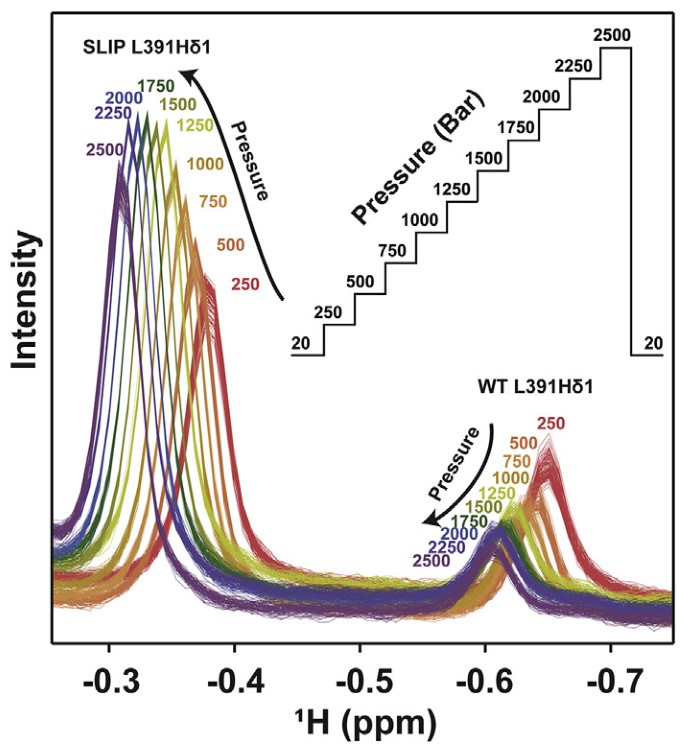 Figure 1. 13C-edited 1D 1H NMR spectra of L391 Hδ1 recorded at pressures between 20 and 2500 bar. (Xu, X.; et al. 2021)
Figure 1. 13C-edited 1D 1H NMR spectra of L391 Hδ1 recorded at pressures between 20 and 2500 bar. (Xu, X.; et al. 2021)
Creative Biostructure has high-end equipment with patented and proprietary design and manufacturing processes, modular design of various accessories for variable temperature, and high-pressure simulations. With high-precision thermostatic probes for more stable data acquisition, our NMR services provide systematic and unique information for a wide range of scientific disciplines.
Service Advantages
- Large-bore NMR analysis and visualization system
- Specialized variable temperature and high-pressure probes
- Ring pressure tracking, pressure accuracy 0.25%
- Providing information not only on the three-dimensional structure of biomolecules but also on their thermodynamic and kinetic properties, making it an essential tool for understanding the function of biomolecules at atomic resolution
- Providing the possibility to monitor structural transformations that occur during protein unfolding at atomic resolution and determining the kinetic properties of the process
- Equipped with modern cryogenic probes to provide unparalleled NMR performance and reliability
Practical Application Examples
- Transition state measurements in enzyme catalysis.
- Characterizing protein functional sub-states and protein structures.
- Understanding the relationship between amino acid sequence, structure, and dynamic properties of the natural conformation of proteins.
- Understanding the details of protein denaturation and partially folded state conformations and detecting intrinsic protein fluctuations.
- Quantitative tests to analyze physical parameters such as pore structure, pore size distribution, and movable/bound fluid saturation in unconventional reservoirs.
- Probing the thermodynamic and kinetic characteristics of proteins interconverting between stable folded conformations.
Creative Biostructure has high-end equipment to provide a full range of high-pressure NMR analysis services to customers in the fields of structural biology and molecular biophysics, helping our customers to obtain unique and in-depth research information. Please feel free to contact us to submit your analytical needs or feedback on our services, and you will receive a satisfactory response soon.
Ordering Process
Reference
- Xu, X.; et al. Volume and compressibility differences between protein conformations revealed by high-pressure NMR. Biophysical Journal. 2021, 120(5): 924-935.
