Determination of Protein Structure and Dynamics
Nuclear magnetic resonance (NMR) spectroscopy is an effective method for analyzing protein structure, interaction and dynamics at atomic resolution and in various sample states (including solution state, solid state and membrane environment). It can also provide rich information about the conformation and interaction dynamics, which occurs on the time scale from picosecond to second or even several days, and the sample state ranges from diluted solution to living cells in various forms.
As an expert in the field of nuclear magnetic resonance, Creative Biostructure applies nuclear magnetic resonance technology to provide customers with analysis services of protein structure and dynamics.
Determination of Protein Structure by NMR
Our general procedure for protein NMR structure determination usually includes four stages
- Preparation of Isotope Labeled Protein Samples
- NMR data collection and analysis, especially the atoms in the chemically shifted protein molecules assigned to 1H, 15N and 13C
- Structural calculations and improvements using distance and/or direction constraints from specific NMR experiments
- Structural quality assessment, each of which has been thoroughly introduced and reviewed. In addition to the structural information derived from the traditional nuclear Overhauser effect (NOE), the paramagnetic relaxation enhancement (PRE) provides a new structural constraint and residual dipole coupling (RDC).
Our Service Scopes
- Protein structure analysis in a complex environment.
- Protein structure analysis in a crowded and confined environment.
- Protein structure analysis in living cells.
- Structural analysis of membrane proteins.
Determination of Protein Dynamics by NMR
Protein dynamics refers to the structural fluctuation or conformational exchange of proteins, which is crucial to protein function. In most cases, the protein will not stay in a static structural state, but will transform between multiple conformational states.
Our Technologies
- Carr-Purcell Meiboom-Gill Relaxation Dispersion, CPMG RD
- Nuclear Spin Relaxation, NSR
- Rotating Frame Relaxation Dispersion, RF RD
- Real-Time NMR, RT NMR
- EXchange SpectroscopY, EXSY (also known as zz-exchange)
- Residual Dipolar Coupling, RDC
- Paramagnetic Relaxation Enhancement, PRE
- Lineshape analysis
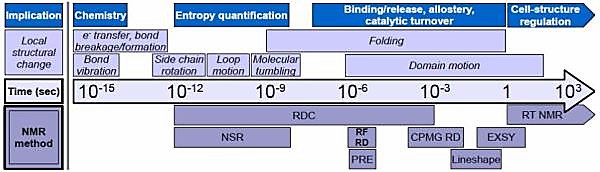 Figure 1. Protein conformational changes over a broad range of timescales enable their biological function (Kleckner & Foster, 2011)
Figure 1. Protein conformational changes over a broad range of timescales enable their biological function (Kleckner & Foster, 2011)
Our Service Scopes
- Nuclear magnetic resonance spectroscopy to explore protein conformation balance.
- Detection of invisible state of protein by relaxation measurement.
- Cellular characterization of protein dynamics.
Related Services
Characterization of Photoreceptor Membrane Proteins
Structure and Dynamics of Membrane-Bound Proteins
Analysis of Soluble Protein Complexes
Hydration and Molecular Dynamics of Collagen
Characterization of Intrinsically Disordered Proteins
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
Reference
- Kleckner I R, Foster M P. An introduction to NMR-based approaches for measuring protein dynamics. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 2011, 1814(8): 942-968.
