Fluorescence Resonance Energy Transfer (FRET) Assay for Coronavirus Research
Interactions between biological macromolecules regulate various biological processes. Many molecular biology and biochemistry methods have been developed to detect this interaction, such as pull-down assay, co-immunoprecipitation (Co-IP), yeast two-hybrid analysis, surface plasmon resonance (SPR) and isothermal titration calorimetry (ITC). However, fluorescence resonance energy transfer (FRET) technology has gained great popularity due to its ability to detect interactions with extremely high spatiotemporal resolution in physiological conditions in a non-invasive manner. As an expert in structural biology and protein research, Creative Biostructure is helping our customers with diverse basic and preclinical research services targeting coronavirus infections, including COVID-19. We offer FRET technology-based assay development and detection services for coronavirus research.
Brief Introduction to FRET Technology
FRET is a process that involves the non-radiative energy transfer from the “donor” fluorophore to the “acceptor” fluorophore. In short, FRET is a distance-dependent interaction between closely adjacent fluorescent donor-acceptor pairs, where the fluorescence energy is transferred non-radiatively from the excited donor to the appropriate receptor molecule. The efficiency of FRET greatly depends on the distance between the donor and the acceptor and on the overlapping spectrum of donor-emission and acceptor-excitation. The FRET phenomenon is very useful for biomedical research because: 1) The excellent sensitivity to molecular distance (1-10 nm) makes FRET a good reporter of near distances and small perturbation in proximity; 2) The distance that occurs closely matches the size of many biomolecules, such as the size of proteins, the distance between sites on the oligomeric protein, and interacting molecules; 3) Although the distance resolution of FRET is far less than that of X-ray crystallography, the advantage of FRET is that it can be applied in physiological conditions in real time.
The advancement of fluorescence microscopy technology combined with the development of new FRET pairs has made the rapid application of FRET technology in biomedical research, such as protein-protein interaction visualization, conformational rearrangement probing, nucleic acid research, gene expression characterization, and FRET-based biosensors.
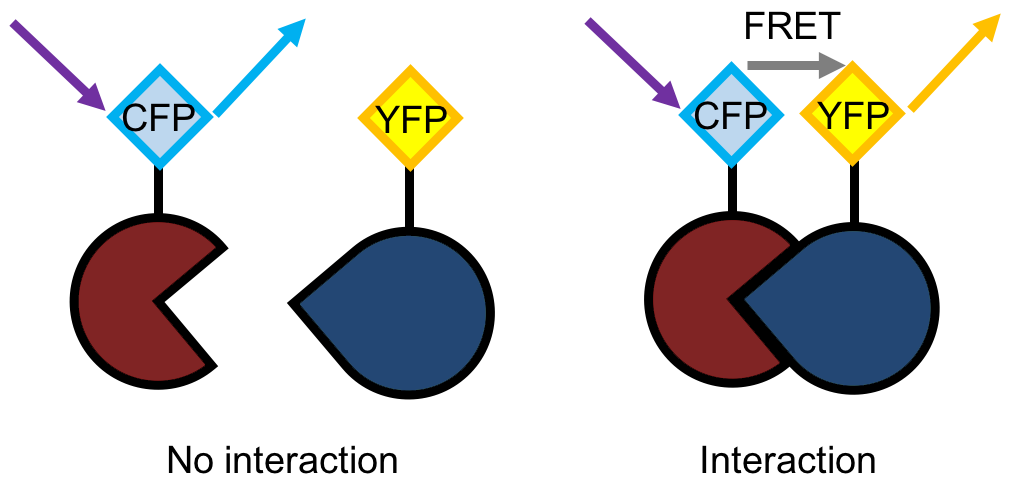 Figure 1. Schematic of the FRET principle.
Figure 1. Schematic of the FRET principle.
Our FRET Services for Coronavirus Research
In previous studies, researchers have developed the FRET-based method to assess proteolytic activity and to explore potential inhibitors of SARS-CoV 3-chymotrypsin-like protease (3CLpro). It is well known that 3CLpro plays a key role in viral replication and is an attractive target for anti-SARS drug discovery. In response to the SARS-CoV-2 pandemic and future coronavirus infection, Creative Biostructure leverages years of experience in contract services based on FRET technology to help you in:
- FRET-based assay development (allowing nanoparticles such as quantum dots to replace traditional fluorophores)
- Analysis of biomolecular interactions involved in coronavirus infection
- Protein trafficking and co-localizations
- Determination of the stoichiometry of protein complexes
- Monitoring protein conformational changes
Creative Biostructure has developed diverse approaches based on advanced technologies for analysis of biomacromolecules interactions. We will meet your specific requirements to protect your scientific investment. If you have any questions, please do not hesitate to contact us. Our customer service representatives are available 24 hours a day from Monday to Sunday.
Contact us to discuss your project!
References
- Ma L.; et al. Application of fluorescence resonance energy transfer in protein studies. Journal of Molecular Structure. 2014, 1077: 87-100.
- Chen S.; et al. Enzymatic activity characterization of SARS coronavirus 3C-like protease by fluorescence resonance energy transfer technique. Acta Pharmacologica Sinica. 2005, 26(1): 99-106.
- Kuang W F.; et al. Mutational and inhibitive analysis of SARS coronavirus 3C-like protease by fluorescence resonance energy transfer-based assays. Biochemical and Biophysical Research Communications. 2005, 331(4): 1554-1559.

 Figure 1. Schematic of the FRET principle.
Figure 1. Schematic of the FRET principle.