Creative Biostructure provides unmatched custom Mempro™ CRAL-TRIO domain-containing protein production services using detergent-free expression system.
CRAL-TRIO domains are the protein structural domains that can bind small lipophilic ligands. The structure of this domain contains several alpha helices as well as a beta sheet composed of 6 strands. Strands 2,3,4 and 5 form a parallel beta sheet with strands 1 and 6 being anti-parallel. CRAL-TRIO domain is originally identified in GTPase-activating proteins (GAPs), a guanine nucleotide exchange factors (GEFs) and a family of hydrophobic ligand binding proteins. TRIO protein involved in coordinating actin remodeling, which is necessary for cell migration and growth. Conventional approaches for CRAL-TRIO domain-containing protein purification need extraction from the membrane into detergent micelles to form detergent-solubilized samples. However, detergent can induce conformational change on membrane proteins. Our alternative detergent-free expression systems can best reserve the native conformation of CRAL-TRIO domain-containing proteins for further structural studies.
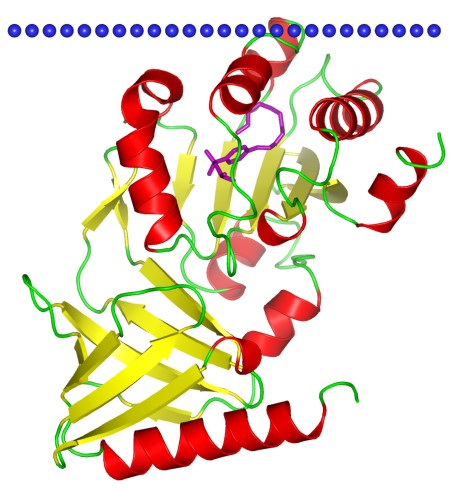
Figure 1. Neurofibromin, a CRAL-TRIO domain-containing protein. (OPM database)
Experters from Creative Biostructure have great experience in high-yield CRAL-TRIO domain-containing protein production using detergent-free membrane protein expression system, we can employ various strategies for Mempro™ detergent-free CRAL-TRIO domain-containing protein production, including:
- Mempro™ CRAL-TRIO domain-containing protein production using nanodiscs
Nanodiscs are the self-assembly system to stabilize membrane proteins removed from the membrane by membrane scaffold proteins (MSPs) in no need of detergent. Creative Biostructure can reconstitute CRAL-TRIO domain-containing proteins into nanodiscs in detergent-free process: Expressing the CRAL-TRIO domain-containing proteins in a cell-free system with the addition of pre-assembled nanodiscs.
- Mempro™ CRAL-TRIO domain-containing protein production using amphipols
The amphipathic amphipols have the ability to “trap” around transmembrane regions of proteins, allowing them to stay folded. Creative Biostructure can utilize A8-35 to solubilize CRAL-TRIO domain-containing proteins during purification step.
- Mempro™ CRAL-TRIO domain-containing protein production using poly (styrene-co-maleic acid) lipid particles (SMALPs)
The SMALPs are self-assembled by the simple addition of the SMA co-polymers. At neutral or alkaline pH, a disc-like structure assembles itself, encapsulating CRAL-TRIO domain-containing proteins in a form amenable to be purified.
These novel detergent-free technologies for CRAL-TRIO domain-containing protein production can be obtained easily, and enabling more comprehensively structural and functional studies.
Creative Biostructure provides other various Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
C. Tribet, et al. (1996). Amphipols: Polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci U. S. A. 93: 15047-15050.
J. M. bomar, et al. (2003). Mutations in a novel gene encoding a CRAL-TRIO domain cause human Cayman ataxia and ataxia/dystonia in the jittery mouse. Nature Gen., 35: 264-269.
K. G. Johnson and K. Kornfeld (2010). The CRAL/TRIO and GOLD domain protein TAP-1 regulates RAF-1 activation. Dev. Biol., 341(2): 464-471.
M. Jamshad, et al. (2011). Surfactant-free purification of membrane proteins with intact native membrane environment. Biochem. Soc. Trans., 39: 813-818.