Secondary Structure Analysis
The secondary structure of proteins describes strands or stretches with unique characteristic local structural conformations based on hydrogen bonds. The two main types of protein secondary structures are α-helix and β-sheet. The α-helix is a right-handed strand in which the side chain of the amino acid sequence extends laterally. Hydrogen bonds are responsible for the structural stability of proteins. The hydrogen bond in a β-sheet fit between the strands (inter-strand). The sheet conformation is composed of pairs of strands lying side by side. The carbonyl oxygens in one strand interact with the amino hydrogens of the adjacent strand.
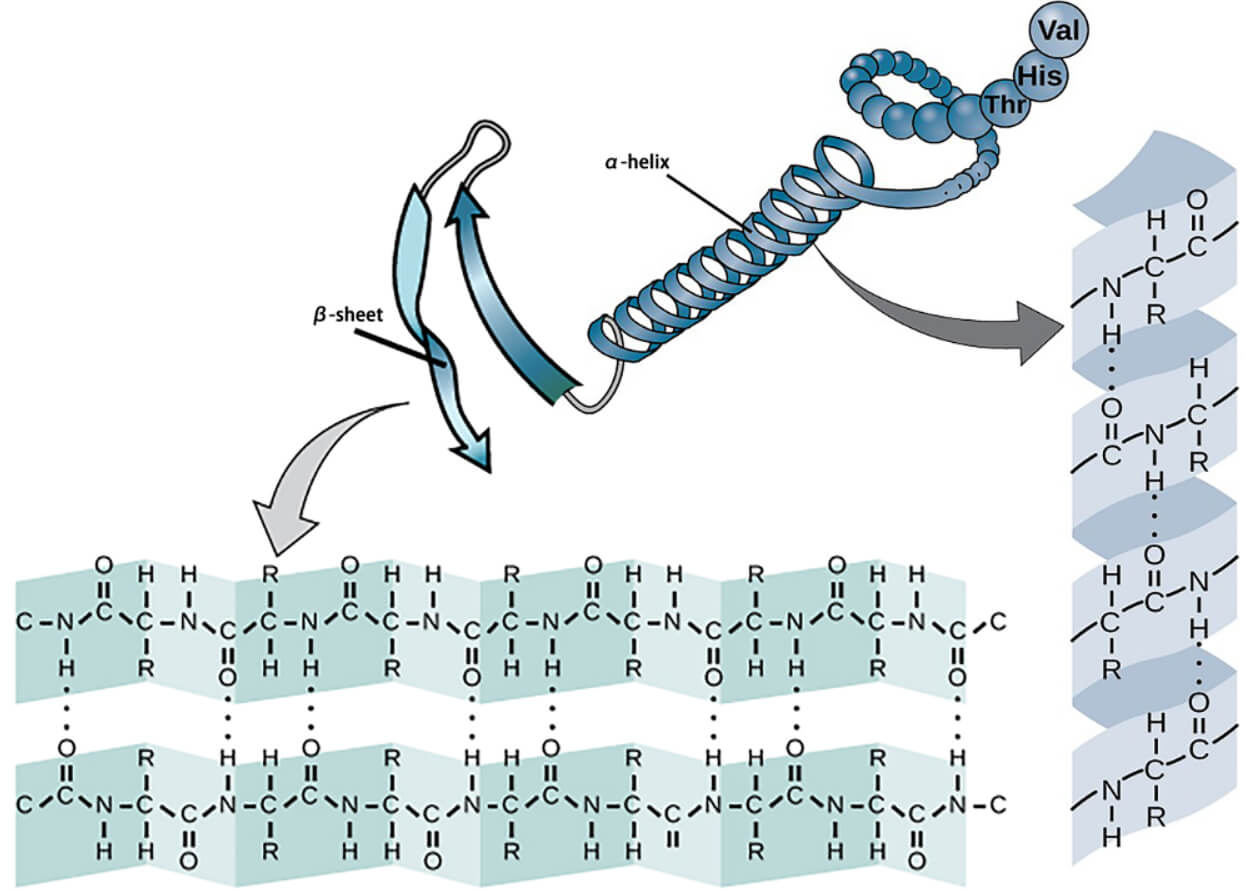
Creative Biostructure provides several services for investigating protein secondary structure. Quantifying the secondary structure components such as α-helix, β-sheet, bends, and turns has been used to better understand the higher-order structure (HOS) product quality attributes of the protein under study and utilized to support stability studies, formulation development, process development, and comparability studies.
Featured Secondary Structure Analysis Services
CD spectroscopy is a technique that has been widely applied in structural biology for examining the secondary structure, tertiary structure, conformational changes, as well as folding and binding interactions involving protein molecules. The CD spectrum arises from an electronically symmetrical chromophore in an asymmetric environment. The chromophores that absorb light in the UV range are the peptide backbone and the aromatic amino acids and disulfide bonds. The result is electromagnetic interactions between neighboring chromophores that can be detected spectroscopically.
The utilize of FTIR spectroscopy to measure the secondary structure of proteins has dramatically expanded in the last few decades, encouraged by the requirement to measure protein secondary structure in samples with diverse physical forms without sample manipulation. FTIR spectroscopy has been applied in measuring the secondary structure of the targeted protein as part of HOS characterization.
Proteins are difficult to characterize due to their relatively large molecular weight, complex structure, and a high degree of heterogeneity, requiring extensive technical expertise and resolution solutions. We have a range of protein characterization analytical solutions that provide broad and in-depth analysis of the structural, physicochemical, and functional aspects of proteins.
If you are interested in our secondary structure analysis services, please feel free to contact us for more information or a detailed quote.
Ordering Process
