Creative Biostructure can offer high quality tailored Mempro™ phox homology (PX) domain-containing protein production services using plant-based expression system.
PX domain is a phosphoinositide-binding domain involved in targeting of proteins to cell membrane, which has a conseved motif of approximately 120 residues in length from human to yeast. It is reported that PX domain is originally identified in p40phox and p47phox domains of neutrophilic NADPH oxidase complex. Membrane proteins containing PX domain play crucial roles in cell signalling, lipid modification, protein sorting, and vesicular/membrane trafficking, such as nexins, phospholipase D, and phosphoinositide-3-kinases, which can bind primarily phosphatidylinositol 3-phosphate [PtdIns(3)P] lipids. In addition, the PX domain-containing proteins can interact with other domains and proteins.
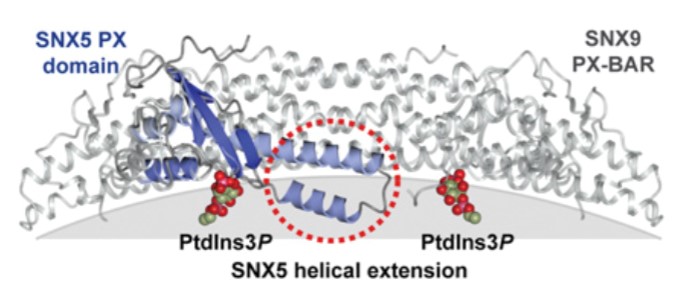
Figure 1. Illustrative structures of the human SNX5 PX domain overlaid with the human PX domain of SNX9 within the SNX9 PX-BAR assembly. (Biochem. J., 2012)
Creative Biostructure has rich professional experience in producing animal-free, low endotoxin and low protease activity membrane proteins relying on Mempro™ plant-based expression platform. Animal-free recombinant proteins are very important for customers concerned with experimental variables caused by trace animal components or mammalian pathogens. Our patent Mempro™ plant-based protein production system can obtain high quality membrane proteins with free of animal components, serum, endotoxins and antibiotics as well as human or animal infectious agents or other endogenous mammalian contamination.
We are able to use different types of plants to produce recombinant membrane proteins, such as Nicotiana benthamiana (tobacco), Medicago rativa (alfalfa), Arabidopsis thaliana (A. thaliana), potato, maize, barley and lettuce. Owing to plant-based expression system has lots of significant advantages, including cost-effective, high yield, easy storage and distribution, friendly environment, and free of infectious or toxic contaminants, this innovative system can be widely utilized in recent years.
With the Mempro™ plant-based expression platform, Creative Biostructure is capable of expressing, isolating, purifying and crystallizing lipocalins to facilitate the study of their biological functions. Misfolding, aggregation, inactivity, poor stability and solubility, etc. are the common difficulties encountered in cell-based expression system. Strikingly, these difficulties can be bypassed in the renewed plant-based expression system.
Creative Biostructure can also provide Mempro™ plant-based virus-like particles (VLPs) production services. Please feel free to contact us for a detailed quote.
References:
A. Wiktorek-Smagur, et al. (2012). Green way of biomedicine – how to force plants to produce new important proteins. Transgenic Plants-Advances and Limitations, Chapter 3. doi: 10.5772/1409.
C. S. Vollert and P. Uetz (2004). The phox homology (PX) domain protein interaction network in yeast. Mol. Cell Proteomics., 3(11):1053-1064.
L. F. Seet and W. Hong (2006). The Phox (PX) domain proteins and membrane traffic. Biochim. Biophy. Acta, 1761(8): 878-896.
R. D. Teasdale and B. M. Collins (2012). Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: structures, functions and roles in disease. Biochem. J., 441: 39-59.
