Case Study 1: TLNTs Suppress Breast Tumor Growth via Apoptosis and Microbiota Modulation (Chen Q, 2023)
A study investigated the anti-tumor potential of tea leaf-derived exosome-like nanotherapeutics (TLNTs) against breast cancer. The TLNTs, with an average size of 166.9 nm and surface charge of -28.8 mV, were effectively internalized by over 80% of 4T1 breast cancer cells within 5 hours. In vitro, TLNTs induced a 2.5-fold increase in intracellular ROS, leading to mitochondrial dysfunction, G0/G1 and S phase arrest, and apoptosis rates of up to 67% after 8 hours.
In vivo experiments showed that orally administered TLNTs significantly reduced tumor volume by 2.2-fold at 3 mg/kg compared to controls, with no observed systemic toxicity. Transcriptomic analysis revealed upregulation of anti-tumor immune genes (e.g., NOS2, CCL4, CXCL9), while gut microbiota profiling indicated restored diversity and reduced abundance of pro-inflammatory bacteria like Oscillibacter and Desulfovibrio. Notably, the anti-tumor efficacy was markedly weakened when antibiotics were used, underscoring the critical role of microbiota in mediating the therapeutic effect.
This study highlights TLNTs as a promising, orally administrable, low-toxicity platform for modulating both tumor biology and the gut-tumor axis.
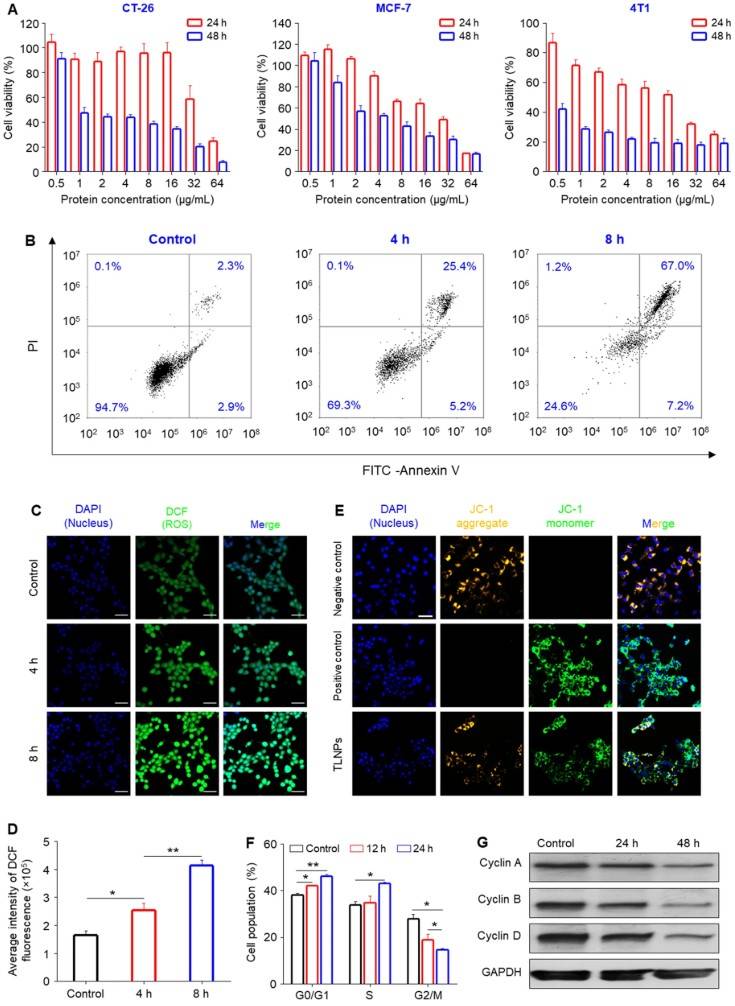 Figure 1. In Vitro Antitumor Activity of TLNTs in 4T1 and Other Tumor Cell Lines. (A) Cell viability assay showing TLNT cytotoxicity in multiple tumor cell lines after 24 and 48 h at increasing protein concentrations (0.5-64 µg/mL). (B) TLNT-induced apoptosis after 4 and 8 h incubation. (C) Confocal images of ROS production in 4T1 cells stained with DCFH-DA after TLNT treatment (scale bar: 50 μm). (D) Quantification of ROS fluorescence intensity. (E) Mitochondrial membrane potential assessment after TLNT exposure (scale bar: 50 μm). (F) Flow cytometry analysis of cell cycle distribution in 4T1 cells after 12 and 24 h TLNT treatment. (G) Western blot showing downregulation of cyclin A, B, and D after 48 h.
Figure 1. In Vitro Antitumor Activity of TLNTs in 4T1 and Other Tumor Cell Lines. (A) Cell viability assay showing TLNT cytotoxicity in multiple tumor cell lines after 24 and 48 h at increasing protein concentrations (0.5-64 µg/mL). (B) TLNT-induced apoptosis after 4 and 8 h incubation. (C) Confocal images of ROS production in 4T1 cells stained with DCFH-DA after TLNT treatment (scale bar: 50 μm). (D) Quantification of ROS fluorescence intensity. (E) Mitochondrial membrane potential assessment after TLNT exposure (scale bar: 50 μm). (F) Flow cytometry analysis of cell cycle distribution in 4T1 cells after 12 and 24 h TLNT treatment. (G) Western blot showing downregulation of cyclin A, B, and D after 48 h.
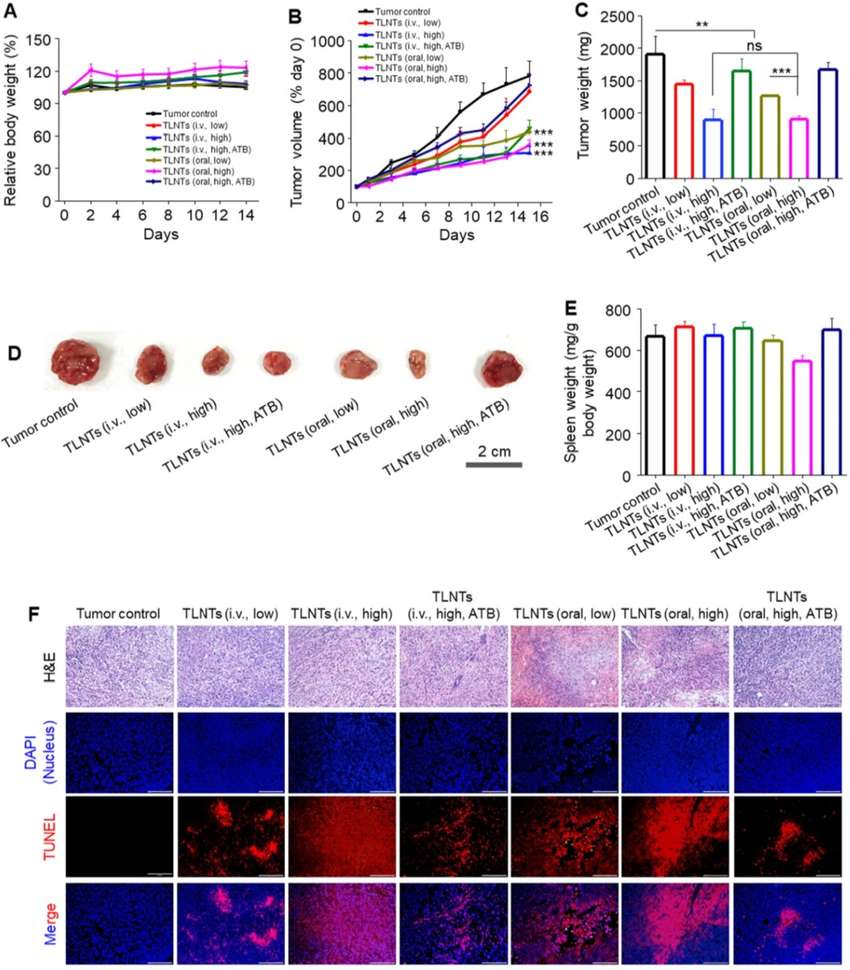 Figure 2. In Vivo Antitumor Efficacy of TLNTs in a Breast Cancer Mouse Model. (A) Body weight changes of tumor-bearing mice throughout the treatment period. (B) Tumor growth curves under different treatment conditions. (C) Final tumor weights measured at endpoint. (D) Representative tumor photographs from each treatment group. (E) Spleen weights of mice after treatment. (F) H&E and TUNEL staining of tumor sections showing histological alterations and apoptosis (scale bar: 100 μm).
Figure 2. In Vivo Antitumor Efficacy of TLNTs in a Breast Cancer Mouse Model. (A) Body weight changes of tumor-bearing mice throughout the treatment period. (B) Tumor growth curves under different treatment conditions. (C) Final tumor weights measured at endpoint. (D) Representative tumor photographs from each treatment group. (E) Spleen weights of mice after treatment. (F) H&E and TUNEL staining of tumor sections showing histological alterations and apoptosis (scale bar: 100 μm).
Case Study 2: TELNs Protect Intestinal Barrier Under Stress Conditions (Gong Q, 2024)
A recent study demonstrated that tea-derived exosome-like nanoparticles (TELNs) can restore intestinal barrier integrity in rats subjected to psychological stress. Oral administration of TELNs (1 mg protein/kg) significantly reduced intestinal permeability, with FITC-dextran uptake and serum endotoxin levels decreased by over 50%.
TELNs upregulated tight junction proteins ZO-1 and occludin, preserving epithelial integrity both in vivo and in LPS-challenged Caco-2 cells, where they also improved transepithelial resistance. Immune barrier function was enhanced via increased IL-22 and Reg3g expression, reducing bacterial translocation across the mucosa.
Importantly, TELN-derived miR-44 and miR-54 boosted ZO-1 expression in a dose-dependent manner, suggesting a role in miRNA-mediated epithelial repair. These findings highlight TELNs as a promising plant-based approach for gut barrier protection under stress.
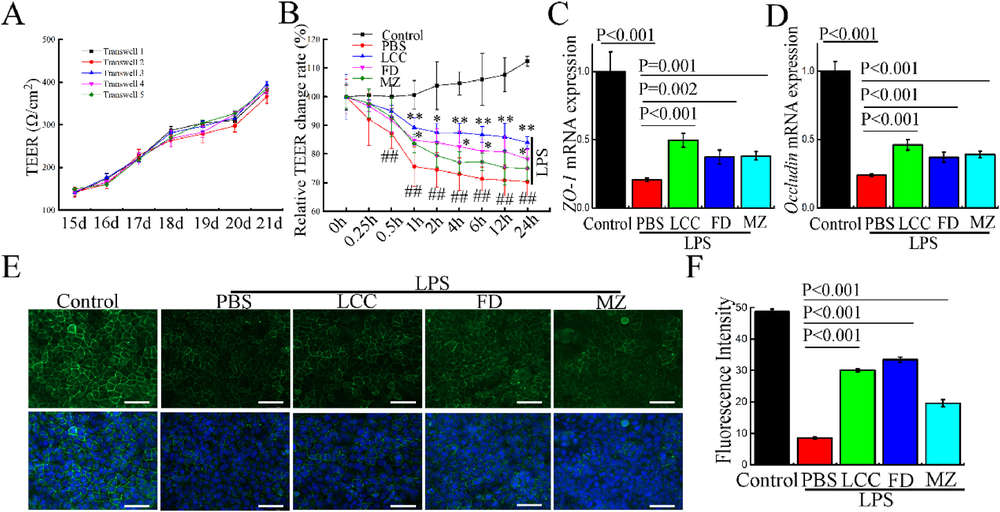 Figure 1. TELNs Protect Epithelial Barrier Integrity in Caco-2 Cells. (A) TEER values of Caco-2 monolayers measured from day 15 to day 21 post-seeding. (B) Relative TEER changes after treatment with different TELNs and LPS stimulation, normalized to initial TEER. (C, D) mRNA expression levels of tight junction markers ZO-1 and Occludin measured by RT-qPCR after TELNs and LPS treatment. Data normalized to control and β-ACTIN used as internal reference. (E) Immunofluorescence images of ZO-1 (green) and nuclei (DAPI, blue) showing junctional localization under different treatments. Scale bar = 50 μm. (F) Quantification of ZO-1 fluorescence intensity from (E) using ImageJ.
Figure 1. TELNs Protect Epithelial Barrier Integrity in Caco-2 Cells. (A) TEER values of Caco-2 monolayers measured from day 15 to day 21 post-seeding. (B) Relative TEER changes after treatment with different TELNs and LPS stimulation, normalized to initial TEER. (C, D) mRNA expression levels of tight junction markers ZO-1 and Occludin measured by RT-qPCR after TELNs and LPS treatment. Data normalized to control and β-ACTIN used as internal reference. (E) Immunofluorescence images of ZO-1 (green) and nuclei (DAPI, blue) showing junctional localization under different treatments. Scale bar = 50 μm. (F) Quantification of ZO-1 fluorescence intensity from (E) using ImageJ.
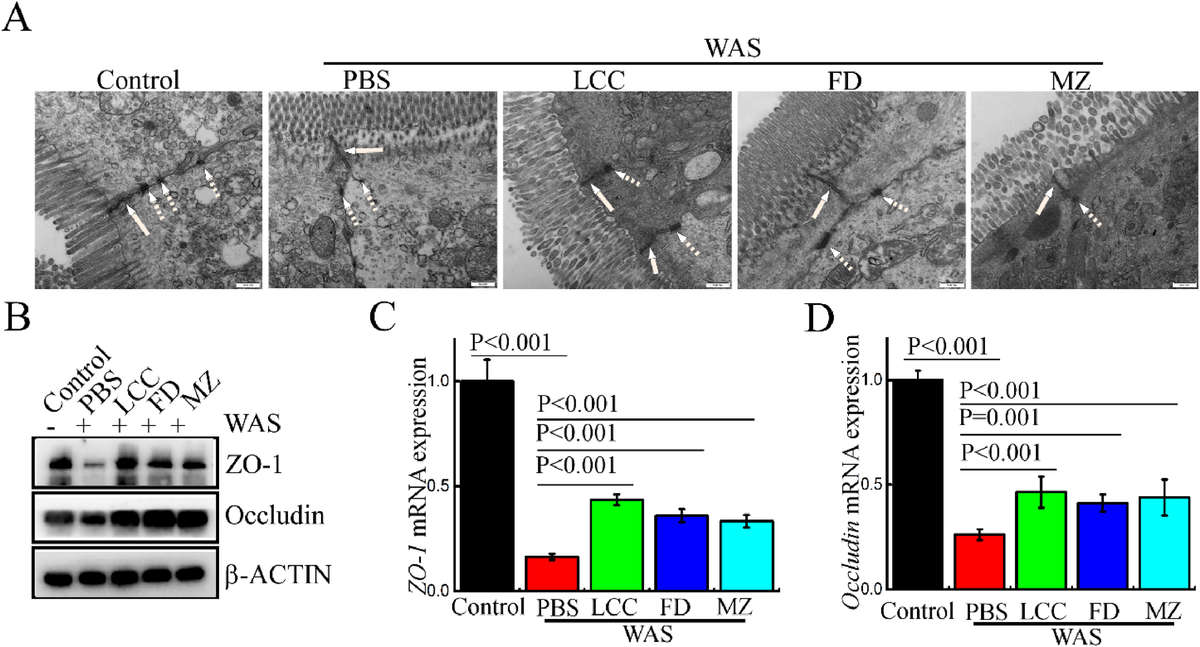 Figure 2. TELNs Reverse WAS-Induced Disruption of Tight Junction Structure. (A) Transmission electron microscopy images showing tight junction (solid arrows) and desmosome (dotted arrows) structures in different treatment groups. Scale bar = 500 nm; magnification: 30,000×. (B) Western blot analysis of tight junction proteins ZO-1 and Occludin in various groups. (C, D) RT-qPCR quantification of ZO-1 (C) and Occludin (D) mRNA levels across treatment groups.
Figure 2. TELNs Reverse WAS-Induced Disruption of Tight Junction Structure. (A) Transmission electron microscopy images showing tight junction (solid arrows) and desmosome (dotted arrows) structures in different treatment groups. Scale bar = 500 nm; magnification: 30,000×. (B) Western blot analysis of tight junction proteins ZO-1 and Occludin in various groups. (C, D) RT-qPCR quantification of ZO-1 (C) and Occludin (D) mRNA levels across treatment groups.
Case Study 3: TELNs Modulate Lipid Metabolism in HepG-2 Cells (Lei X, 2025)
A recent study demonstrated that tea leaf-derived exosome-like nanoparticles (TELNs) can improve lipid metabolism in oleic acid-treated HepG-2 cells. TELNs (~249 nm, -20.6 mV) were efficiently internalized and showed no cytotoxicity up to 300 µg/mL.
Treatment with TELNs significantly reduced lipid accumulation, decreased TG, TC, and LDL-C levels, and increased HDL-C. Liver injury markers ALT and AST were also lowered. Mechanistic analysis revealed that TELNs suppressed miR-21-5p, miR-17-3p, and miR-107, leading to upregulation of their target genes PPAR-α, CYP7A1, and CPT1A, key regulators of fatty acid oxidation. These findings suggest TELNs hold promise as safe, plant-derived modulators of lipid metabolism through miRNA-mediated pathways.
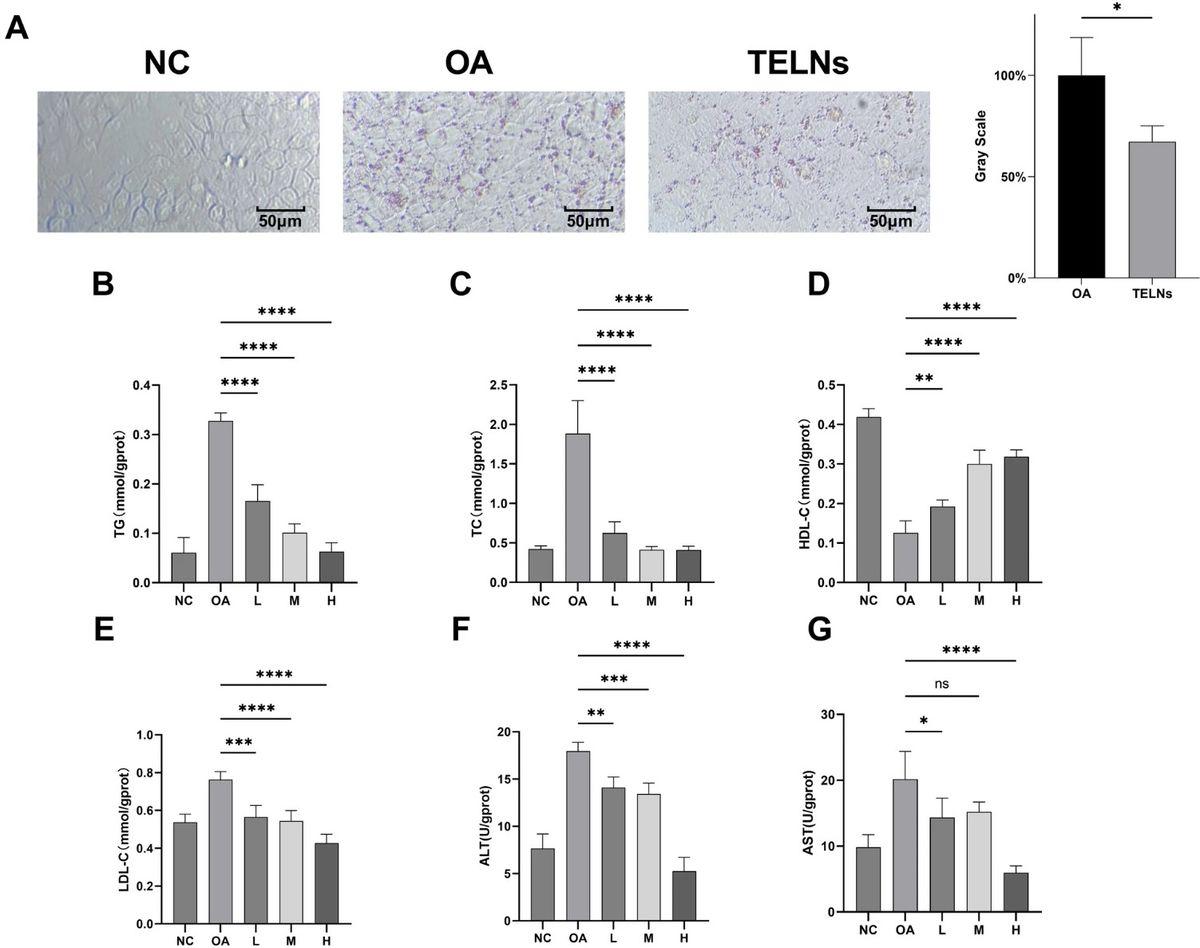 Figure 1. TELNs Regulate Lipid Metabolism in HepG-2 Cells. (A) Oil Red O staining and grayscale analysis show that TELNs (300 μg/mL) reduce OA-induced lipid accumulation in HepG-2 cells. Red oil droplets were abundant in the OA group and significantly reduced in the TELNs group. (B-G) Dose-dependent effects of TELNs (100, 200, 300 μg/mL) on triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels.
Figure 1. TELNs Regulate Lipid Metabolism in HepG-2 Cells. (A) Oil Red O staining and grayscale analysis show that TELNs (300 μg/mL) reduce OA-induced lipid accumulation in HepG-2 cells. Red oil droplets were abundant in the OA group and significantly reduced in the TELNs group. (B-G) Dose-dependent effects of TELNs (100, 200, 300 μg/mL) on triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels.
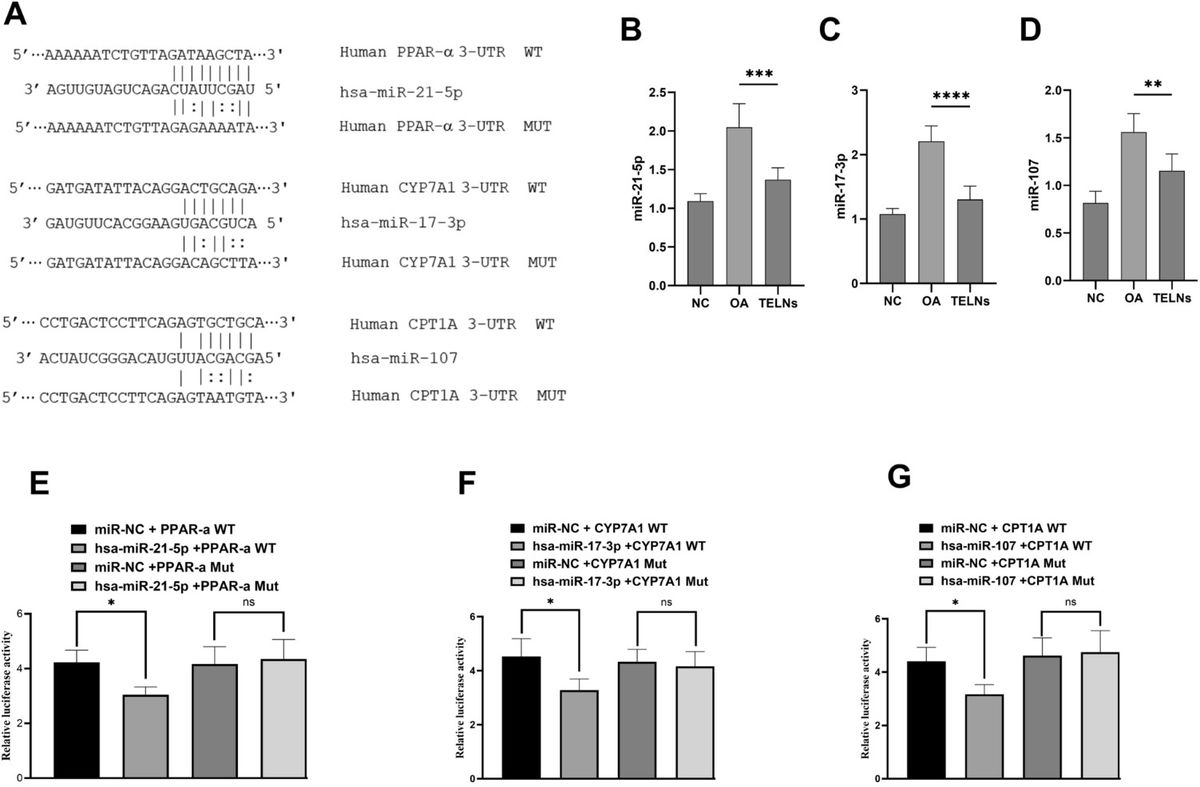 Figure 2. TELNs Regulate Lipid Metabolism Genes via miRNA Interactions. (A) Predicted binding sites between miR-21-5p, miR-17-3p, and miR-107 and their lipid metabolism-related targets: PPAR-α, CYP7A1, and CPT1A. (B-D) TELNs treatment (300 μg/mL) significantly reduced expression of these miRNAs in HepG-2 cells compared to OA group. (E, F) Dual-luciferase reporter assays confirmed that the three miRNAs directly target the respective genes. Mutation of binding sites abolished the suppression effect.
Figure 2. TELNs Regulate Lipid Metabolism Genes via miRNA Interactions. (A) Predicted binding sites between miR-21-5p, miR-17-3p, and miR-107 and their lipid metabolism-related targets: PPAR-α, CYP7A1, and CPT1A. (B-D) TELNs treatment (300 μg/mL) significantly reduced expression of these miRNAs in HepG-2 cells compared to OA group. (E, F) Dual-luciferase reporter assays confirmed that the three miRNAs directly target the respective genes. Mutation of binding sites abolished the suppression effect.
References
- Chen Q, Zu M, Gong H, et al. Tea leaf-derived exosome-like nanotherapeutics retard breast tumor growth by pro-apoptosis and microbiota modulation. Journal of Nanobiotechnology. 2023, 21(1): 6.
- Gong Q, Sun Y, Liu L, et al. Oral administration of tea-derived exosome-like nanoparticles protects epithelial and immune barrier of intestine from psychological stress. Heliyon. 2024, 10(17).
- Lei X, Li H, Chen S, et al. Tea leaf exosome-like nanoparticles (TELNs) improve oleic acid-induced lipid metabolism by regulating miRNAs in HepG-2 cells. Bioresources and Bioprocessing. 2025, 12(1): 9.
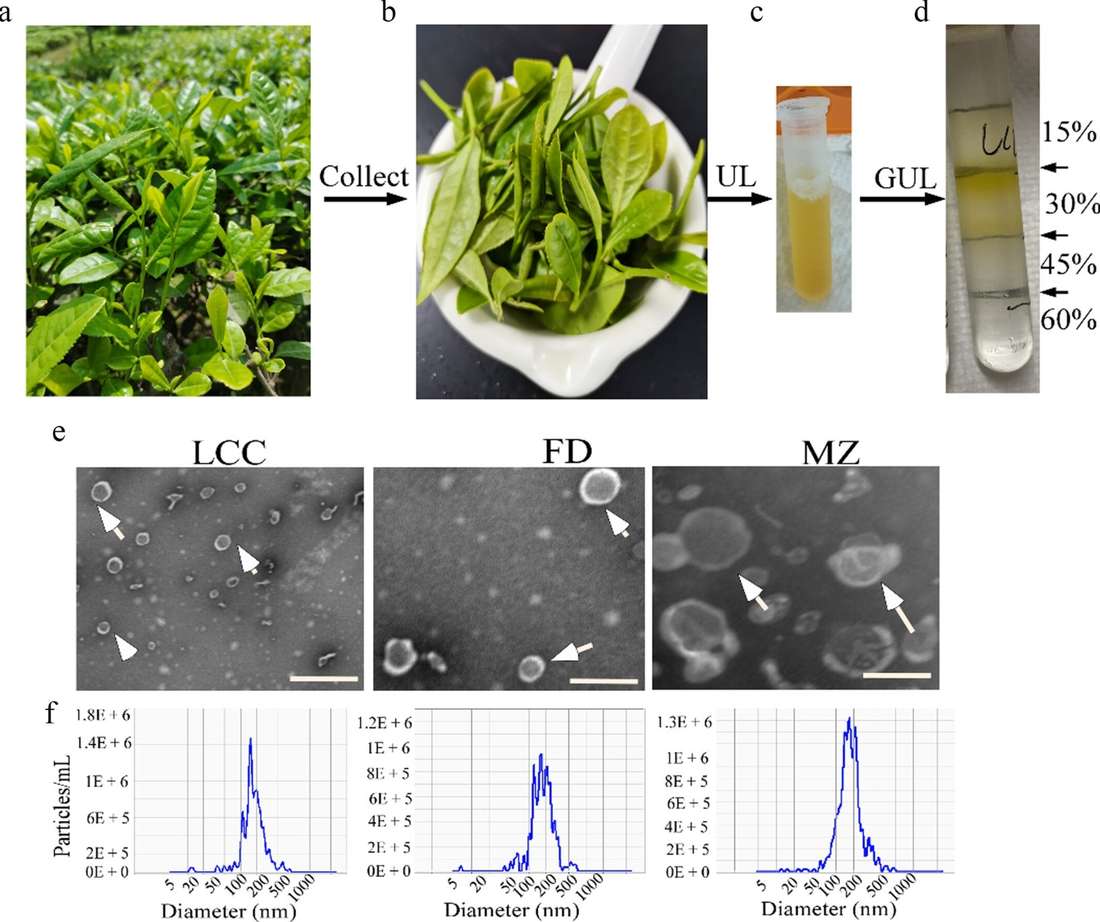 Isolation and Characterization of TELNs. (a-d) TELNs were isolated from ground tea leaf juice by ultracentrifugation (UL) and gradient ultracentrifugation (GUL), with TELNs found at the 15/30% interface. (e) Representative TEM image of TELNs; white arrows indicate vesicle structures. Magnification: 50,000×; scale bar: 200 nm. (f) NTA analysis of TELNs showing particle size distribution. (Gong Q, et al., 2024)
Isolation and Characterization of TELNs. (a-d) TELNs were isolated from ground tea leaf juice by ultracentrifugation (UL) and gradient ultracentrifugation (GUL), with TELNs found at the 15/30% interface. (e) Representative TEM image of TELNs; white arrows indicate vesicle structures. Magnification: 50,000×; scale bar: 200 nm. (f) NTA analysis of TELNs showing particle size distribution. (Gong Q, et al., 2024) Figure 1. In Vitro Antitumor Activity of TLNTs in 4T1 and Other Tumor Cell Lines. (A) Cell viability assay showing TLNT cytotoxicity in multiple tumor cell lines after 24 and 48 h at increasing protein concentrations (0.5-64 µg/mL). (B) TLNT-induced apoptosis after 4 and 8 h incubation. (C) Confocal images of ROS production in 4T1 cells stained with DCFH-DA after TLNT treatment (scale bar: 50 μm). (D) Quantification of ROS fluorescence intensity. (E) Mitochondrial membrane potential assessment after TLNT exposure (scale bar: 50 μm). (F) Flow cytometry analysis of cell cycle distribution in 4T1 cells after 12 and 24 h TLNT treatment. (G) Western blot showing downregulation of cyclin A, B, and D after 48 h.
Figure 1. In Vitro Antitumor Activity of TLNTs in 4T1 and Other Tumor Cell Lines. (A) Cell viability assay showing TLNT cytotoxicity in multiple tumor cell lines after 24 and 48 h at increasing protein concentrations (0.5-64 µg/mL). (B) TLNT-induced apoptosis after 4 and 8 h incubation. (C) Confocal images of ROS production in 4T1 cells stained with DCFH-DA after TLNT treatment (scale bar: 50 μm). (D) Quantification of ROS fluorescence intensity. (E) Mitochondrial membrane potential assessment after TLNT exposure (scale bar: 50 μm). (F) Flow cytometry analysis of cell cycle distribution in 4T1 cells after 12 and 24 h TLNT treatment. (G) Western blot showing downregulation of cyclin A, B, and D after 48 h. Figure 2. In Vivo Antitumor Efficacy of TLNTs in a Breast Cancer Mouse Model. (A) Body weight changes of tumor-bearing mice throughout the treatment period. (B) Tumor growth curves under different treatment conditions. (C) Final tumor weights measured at endpoint. (D) Representative tumor photographs from each treatment group. (E) Spleen weights of mice after treatment. (F) H&E and TUNEL staining of tumor sections showing histological alterations and apoptosis (scale bar: 100 μm).
Figure 2. In Vivo Antitumor Efficacy of TLNTs in a Breast Cancer Mouse Model. (A) Body weight changes of tumor-bearing mice throughout the treatment period. (B) Tumor growth curves under different treatment conditions. (C) Final tumor weights measured at endpoint. (D) Representative tumor photographs from each treatment group. (E) Spleen weights of mice after treatment. (F) H&E and TUNEL staining of tumor sections showing histological alterations and apoptosis (scale bar: 100 μm). Figure 1. TELNs Protect Epithelial Barrier Integrity in Caco-2 Cells. (A) TEER values of Caco-2 monolayers measured from day 15 to day 21 post-seeding. (B) Relative TEER changes after treatment with different TELNs and LPS stimulation, normalized to initial TEER. (C, D) mRNA expression levels of tight junction markers ZO-1 and Occludin measured by RT-qPCR after TELNs and LPS treatment. Data normalized to control and β-ACTIN used as internal reference. (E) Immunofluorescence images of ZO-1 (green) and nuclei (DAPI, blue) showing junctional localization under different treatments. Scale bar = 50 μm. (F) Quantification of ZO-1 fluorescence intensity from (E) using ImageJ.
Figure 1. TELNs Protect Epithelial Barrier Integrity in Caco-2 Cells. (A) TEER values of Caco-2 monolayers measured from day 15 to day 21 post-seeding. (B) Relative TEER changes after treatment with different TELNs and LPS stimulation, normalized to initial TEER. (C, D) mRNA expression levels of tight junction markers ZO-1 and Occludin measured by RT-qPCR after TELNs and LPS treatment. Data normalized to control and β-ACTIN used as internal reference. (E) Immunofluorescence images of ZO-1 (green) and nuclei (DAPI, blue) showing junctional localization under different treatments. Scale bar = 50 μm. (F) Quantification of ZO-1 fluorescence intensity from (E) using ImageJ. Figure 2. TELNs Reverse WAS-Induced Disruption of Tight Junction Structure. (A) Transmission electron microscopy images showing tight junction (solid arrows) and desmosome (dotted arrows) structures in different treatment groups. Scale bar = 500 nm; magnification: 30,000×. (B) Western blot analysis of tight junction proteins ZO-1 and Occludin in various groups. (C, D) RT-qPCR quantification of ZO-1 (C) and Occludin (D) mRNA levels across treatment groups.
Figure 2. TELNs Reverse WAS-Induced Disruption of Tight Junction Structure. (A) Transmission electron microscopy images showing tight junction (solid arrows) and desmosome (dotted arrows) structures in different treatment groups. Scale bar = 500 nm; magnification: 30,000×. (B) Western blot analysis of tight junction proteins ZO-1 and Occludin in various groups. (C, D) RT-qPCR quantification of ZO-1 (C) and Occludin (D) mRNA levels across treatment groups. Figure 1. TELNs Regulate Lipid Metabolism in HepG-2 Cells. (A) Oil Red O staining and grayscale analysis show that TELNs (300 μg/mL) reduce OA-induced lipid accumulation in HepG-2 cells. Red oil droplets were abundant in the OA group and significantly reduced in the TELNs group. (B-G) Dose-dependent effects of TELNs (100, 200, 300 μg/mL) on triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels.
Figure 1. TELNs Regulate Lipid Metabolism in HepG-2 Cells. (A) Oil Red O staining and grayscale analysis show that TELNs (300 μg/mL) reduce OA-induced lipid accumulation in HepG-2 cells. Red oil droplets were abundant in the OA group and significantly reduced in the TELNs group. (B-G) Dose-dependent effects of TELNs (100, 200, 300 μg/mL) on triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels. Figure 2. TELNs Regulate Lipid Metabolism Genes via miRNA Interactions. (A) Predicted binding sites between miR-21-5p, miR-17-3p, and miR-107 and their lipid metabolism-related targets: PPAR-α, CYP7A1, and CPT1A. (B-D) TELNs treatment (300 μg/mL) significantly reduced expression of these miRNAs in HepG-2 cells compared to OA group. (E, F) Dual-luciferase reporter assays confirmed that the three miRNAs directly target the respective genes. Mutation of binding sites abolished the suppression effect.
Figure 2. TELNs Regulate Lipid Metabolism Genes via miRNA Interactions. (A) Predicted binding sites between miR-21-5p, miR-17-3p, and miR-107 and their lipid metabolism-related targets: PPAR-α, CYP7A1, and CPT1A. (B-D) TELNs treatment (300 μg/mL) significantly reduced expression of these miRNAs in HepG-2 cells compared to OA group. (E, F) Dual-luciferase reporter assays confirmed that the three miRNAs directly target the respective genes. Mutation of binding sites abolished the suppression effect.