PNExo™ Exosome-Rose(PNE-FRO75)
| Name | PNExo™ Exosome-Rose |
| Cat No. | PNE-FRO75 |
| Source | Rose |
| Product Overview | PNExo™ Exosome Series (Exosomes isolated from Nuts/Seeds) are nanosized (30-150 nm) membrane vesicles extracted from a variety of nuts and seeds, rich in bioactive molecules and proteins. These naturally derived nanoparticles contain a variety of bioactive molecules and proteins, which have been proven to offer numerous benefits in skincare, food enhancement, and health supplement development. Seed exosomes, with their antioxidant, anti-inflammatory, and anti-aging properties, have become an attractive option for the development of innovative products across various industries. PNExo™ is dedicated to the production and delivery of high-quality seed-derived exosome products. Our products undergo a rigorous screening and purification process to ensure their high purity and activity. We can provide both lyophilized powder and frozen liquid according to customer requirements. Lyophilized powder is beneficial for long-term storage at 4°C, while frozen liquid should be maintained at temperatures between -20°C and -80°C. Ultracentrifugation, PEG precipitation, and Tangential Flow Filtration (TFF) technology are utilized for the isolation and production of exosomes, ensuring the highest quality and purity. Creative Biostructure PNExo™ Exosome products guarantee higher purity and quality, and we can provide exosome GMP production and CDMO services to meet our customers' research and production needs. Our commitment to excellence ensures that our seed exosome products are at the forefront of innovation in the cosmetics, food, and health supplement industries. |
| Form | Lyophilized powder / Frozen Liquid |
| Concentration | > 1x10^10 particles |
| Storage | Lyophilized powder store at 4 °C. Frozen liquid store at -20°C to -80°C. Recommended to avoid repeated freeze-and-thaw cycles. |
| Reconstitution | Reconstitute lyophilized exosome by adding deionized water for a desired final concentration. Centrifuge before opening to ensure exosomes are at bottom, resuspend exosomes by pipetting and/or vortex, please avoid bubbles. Centrifuge again and mix well for using. |
| Efficacy | Antioxidant, anti-inflammatory, brightening, reducing melanin production, lightening skin tone, repairing damaged skin, and promoting skin regeneration. |
In addition to supplying high-quality rose-derived exosomes, Creative Biostructure supports your innovation pipeline with a full suite of customized services. We offer advanced plant exosome isolation, profiling, and content validation using industry-leading technologies. For partners with formulation or scale-up needs, our team provides GMP-compliant production and flexible CDMO solutions, enabling seamless transition from research-grade material to commercial readiness. Whether your focus is mechanistic research, ingredient development, or regulatory-aligned manufacturing, our plant exosome platform is built to adapt to your objectives at every stage.
Online Inquiry
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
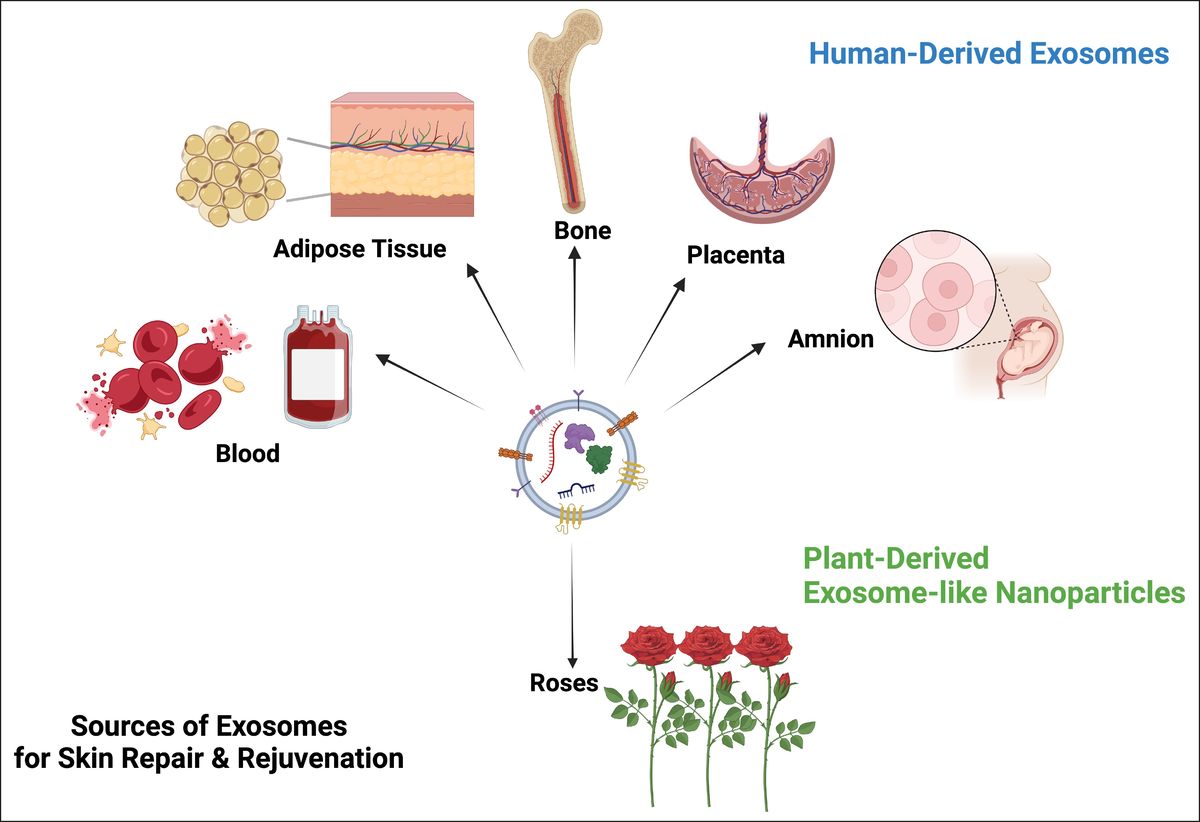 Global Sources of Exosome-Derived Products Used in Skin Rejuvenation. (Theodorakopoulou E, et al., 2024)
Global Sources of Exosome-Derived Products Used in Skin Rejuvenation. (Theodorakopoulou E, et al., 2024)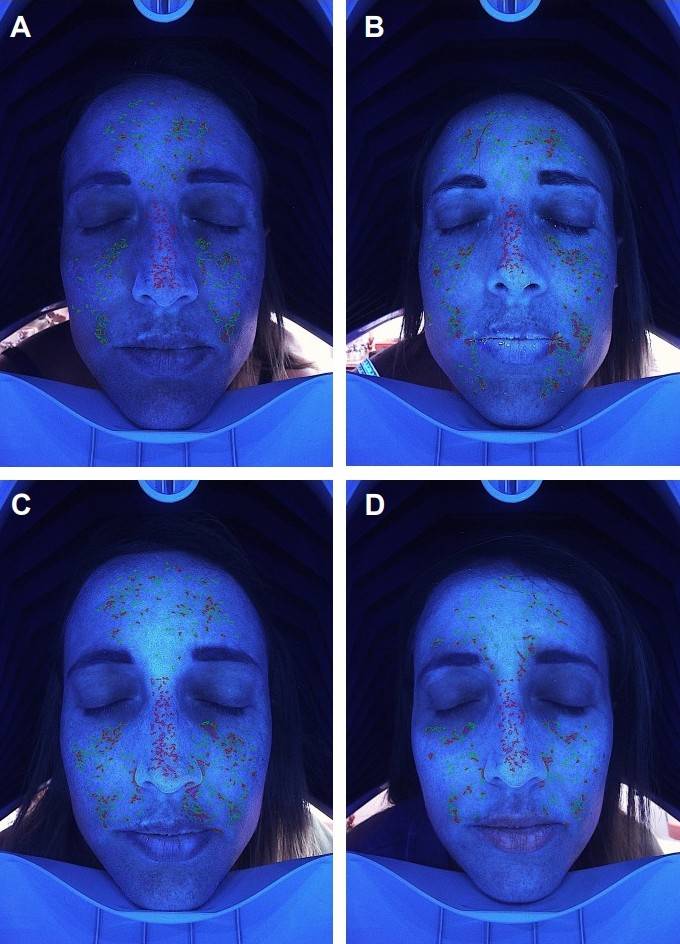 Figure 1. Imaging Assessment of Deep Pigmentation Improvement After RSCE Treatment. Imaging analysis of a 44-year-old female patient showing facial pigmentation changes. (A) Initial distribution of deep pigmentation before RSCE treatment. (B-D) Progressive improvement of pigmentation at Week 3, Week 6, and Week 12 post-treatment, respectively.
Figure 1. Imaging Assessment of Deep Pigmentation Improvement After RSCE Treatment. Imaging analysis of a 44-year-old female patient showing facial pigmentation changes. (A) Initial distribution of deep pigmentation before RSCE treatment. (B-D) Progressive improvement of pigmentation at Week 3, Week 6, and Week 12 post-treatment, respectively.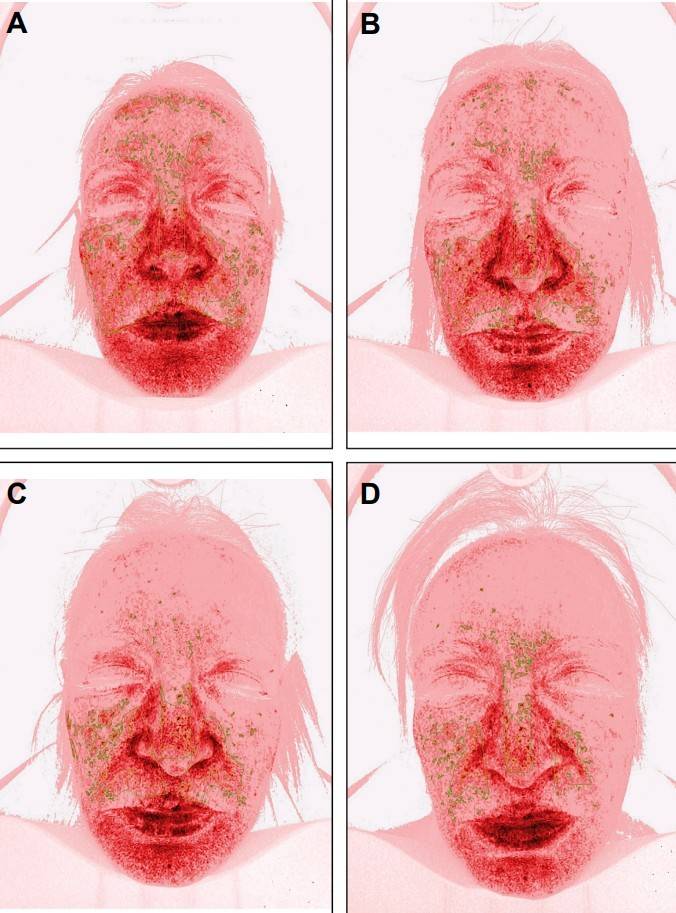 Figure 2. Imaging Assessment of Sensitive Skin Improvement Following RSCE Treatment. Imaging analysis of a 50-year-old female patient with sensitive skin prone to rosacea, inflammation, and telangiectasia. (A) Baseline image showing the intensity and distribution of skin sensitivity. (B-D) Progressive reduction in skin sensitivity at Week 3, Week 6, and Week 12 after treatment.
Figure 2. Imaging Assessment of Sensitive Skin Improvement Following RSCE Treatment. Imaging analysis of a 50-year-old female patient with sensitive skin prone to rosacea, inflammation, and telangiectasia. (A) Baseline image showing the intensity and distribution of skin sensitivity. (B-D) Progressive reduction in skin sensitivity at Week 3, Week 6, and Week 12 after treatment.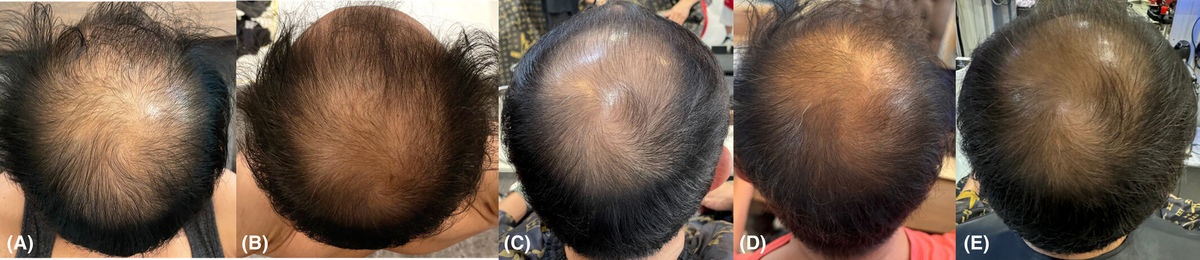 Figure 1. Clinical Outcomes of Exosome Therapy for Androgenetic Alopecia. (A) Baseline showing diffuse scalp hair thinning and frontal-temporal hairline recession. (B) After the 3rd treatment session: visible improvements in hair density, reduced shedding, and increased thickness. (C-D) Continued improvement seen after the 6th and 12th sessions with exosome therapy and electroporation. (E) Sustained hair regrowth and density enhancement observed 3 months post-treatment.
Figure 1. Clinical Outcomes of Exosome Therapy for Androgenetic Alopecia. (A) Baseline showing diffuse scalp hair thinning and frontal-temporal hairline recession. (B) After the 3rd treatment session: visible improvements in hair density, reduced shedding, and increased thickness. (C-D) Continued improvement seen after the 6th and 12th sessions with exosome therapy and electroporation. (E) Sustained hair regrowth and density enhancement observed 3 months post-treatment.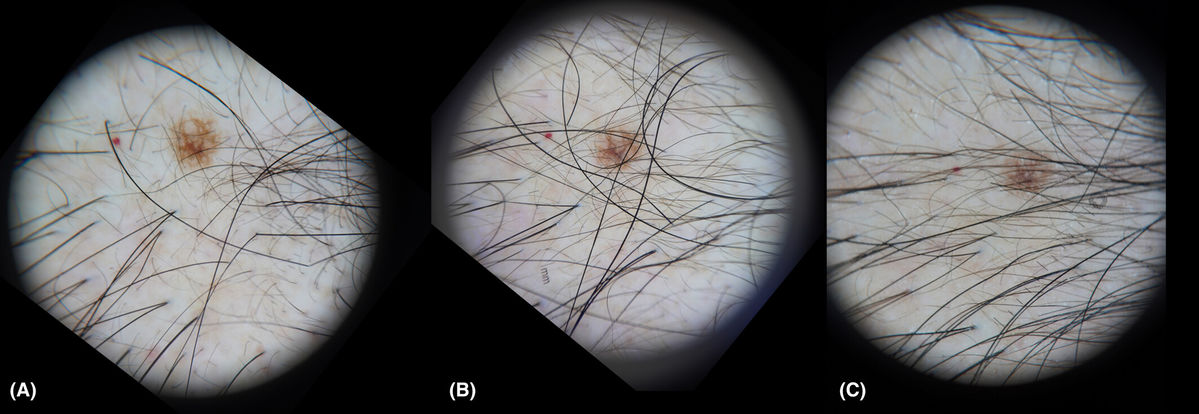 Figure 2. Dermoscopic Evaluation of Mid-Scalp Area During Exosome Therapy. Dermoscopic images of the mid-scalp region taken at three time points: (A) Baseline before treatment, (B) After the 6th session, (C) After the 12th session of exosome electroporation therapy. A nevus and cherry angioma serve as consistent reference markers. No serious adverse events were reported, and the treatment was well-tolerated.
Figure 2. Dermoscopic Evaluation of Mid-Scalp Area During Exosome Therapy. Dermoscopic images of the mid-scalp region taken at three time points: (A) Baseline before treatment, (B) After the 6th session, (C) After the 12th session of exosome electroporation therapy. A nevus and cherry angioma serve as consistent reference markers. No serious adverse events were reported, and the treatment was well-tolerated.