Nuclear Magnetic Resonance (NMR) spectroscopy is one of the most powerful non-destructive analytical techniques in chemistry and structural biology. From elucidating the molecular architecture of complex organic compounds to enabling real-time metabolic monitoring, NMR plays an essential role in chemical analysis. What sets NMR apart is its remarkable precision in quantifying molecular structures, dynamics, interactions, and even reaction pathways. This article examines the principles of NMR, its instrumentation, the factors that influence analytical accuracy, and how recent developments in NMR technology are improving the precision of chemical analysis in a wide range of scientific and industrial fields.

Introduction to Chemical Analysis
Chemical analysis is at the heart of chemistry, pharmacology, materials science, and biochemistry. The ability not only to detect substances, but also to characterize them accurately and reproducibly is critical. In a landscape of tools that includes mass spectrometry, infrared spectroscopy, and chromatography, NMR spectroscopy stands out for its unique ability to provide detailed information about molecular structure and dynamics—without destroying the sample.
Since its inception in the 1940s, NMR has undergone major refinements in magnet technology, radio frequency detection, pulse sequences, and data processing algorithms. Together, these advances have pushed the limits of resolution, sensitivity, and accuracy. But how exactly does NMR improve the accuracy of chemical analysis? That's what we're about to unpack.
Fundamental Principles of NMR
The Spin Behind the Magic
NMR is based on the magnetic properties of certain atomic nuclei. Nuclei with a non-zero spin quantum number (e.g., ¹H, ¹³C, ¹⁵N, ³¹P) generate a magnetic moment. When placed in an external magnetic field, these nuclear spins can align with or against the field, creating discrete energy levels.
 Figure 1. Mechanism of NMR. (Adapted from Han and Wang, 2021)
Figure 1. Mechanism of NMR. (Adapted from Han and Wang, 2021)
Applying a radiofrequency (RF) pulse at the resonance frequency specific to a given nucleus causes a transition between these spin states. The nucleus absorbs energy, flips its spin, and when the RF pulse is removed, relaxes back to its equilibrium state, releasing the absorbed energy. This signal is detected and Fourier transformed into an NMR spectrum.
The Chemical Shift: NMR's Fingerprint
Each nucleus experiences a slightly different magnetic environment depending on its electron density and bonding—this is the chemical shift, typically measured in parts per million (ppm). Chemical shifts provide a highly sensitive measure of the local chemical environment, helping to identify atom types, bonding patterns, and molecular geometry.
 Figure 2. 31P NMR chemical shift ranges (on the ppm scale) and chemical structures of common P functional groups observed in natural samples (Sannigrahi and Ingall, 2005)
Figure 2. 31P NMR chemical shift ranges (on the ppm scale) and chemical structures of common P functional groups observed in natural samples (Sannigrahi and Ingall, 2005)
NMR vs. Traditional Analytical Techniques
Comparison with Mass Spectrometry
Mass spectrometry (MS) is excellent at determining molecular weights and fragmentation patterns. However, it often falls short in resolving isomers—compounds with the same formula but different structures. NMR, on the other hand, can distinguish between isomers based on chemical shifts and coupling constants.
For example, MS cannot readily distinguish between glucose and fructose, whereas NMR readily identifies their structural differences through characteristic chemical shifts and coupling patterns.
Comparison with Chromatography
Chromatographic techniques such as HPLC and GC are invaluable for separating and quantifying compounds. However, they require standards for identification and often lack structural insight. NMR directly identifies compounds without the need for prior separation or reference standards, improving analysis reliability, especially in mixtures.
Synergy with Other Techniques
Importantly, NMR complements rather than competes with MS and chromatography. Techniques such as LC-NMR and MS-NMR hyphenation combine the strengths of each method, resulting in improved accuracy in complex matrices.
 Figure 3. The instrumental setup of LC–NMR system. (Gebretsadik, 2021)
Figure 3. The instrumental setup of LC–NMR system. (Gebretsadik, 2021)
Components of an NMR Spectrometer and Their Roles in Accuracy
High-precision NMR spectroscopy depends on the flawless orchestration of several key components. Each part of the instrument—from magnetic field generation to data interpretation-plays a critical role in delivering accurate, reproducible results. Let's explore how each element contributes to analytical performance:
Superconducting Magnet
The superconducting magnet is the heart of the NMR spectrometer. It generates the strong, stable magnetic field needed to align the nuclear spins in the sample.
- Field Strength: A stronger magnetic field increases the energy gap between spin states, thereby increasing sensitivity and improving spectral resolution. This is especially important when analyzing complex mixtures or low abundance compounds.
- Homogeneity and Stability: Accurate results require a perfectly uniform magnetic field across the sample. Modern instruments use active shimming—the fine adjustment of shim coils-to compensate for minute irregularities. Temperature control systems further stabilize the magnet to reduce frequency drift and ensure consistent results over time.
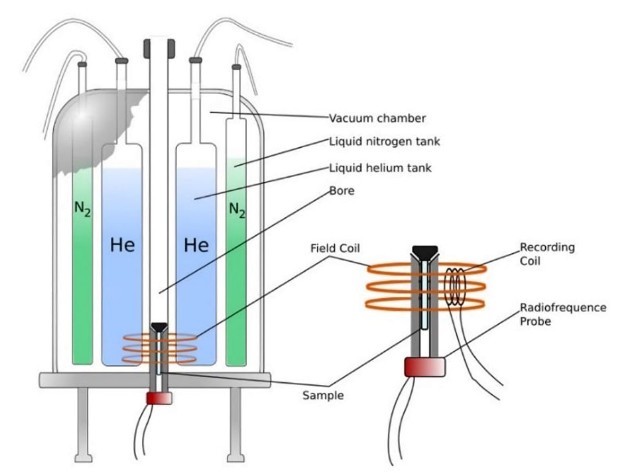 Figure 4. Internal components of a superconducting magnet for an NMR spectrometer, including a detailed view of the probe. (Liu et al., 2024)
Figure 4. Internal components of a superconducting magnet for an NMR spectrometer, including a detailed view of the probe. (Liu et al., 2024)
RF Transmitter and Probe
This subsystem is responsible for delivering the RF pulses that excite the nuclear spins and for detecting the resulting signals.
- Precise Tuning and Matching: The RF transmitter must be tuned to the exact Larmor frequency of the nucleus under study. Mismatched tuning can result in weak excitation and poor signal quality.
- The Probe: This is where the RF coils interact directly with the sample. Probe design—including coil geometry and material—greatly affects the sensitivity and resolution.
- Cryogenic Probes: Advanced cryoprobes cool the RF detection coils to reduce thermal noise, significantly increasing signal-to-noise ratio. This results in sharper peaks and the ability to detect analytes at lower concentrations.
Receiver and Analog-to-Digital Converter (ADC)
Once the nuclei emit RF signals (free induction decay), they are picked up by the receiver and converted into digital data.
- Low-Noise Preamplifiers: These amplify the weak NMR signal without adding significant noise, preserving accuracy.
- High-Resolution ADCs: These devices digitize the signal at high bit depths and sampling rates. The better the digitization, the more accurately the signal can be processed and interpreted.
Data Processor and Software
The final step in NMR involves translating the raw data into meaningful spectra.
- Fourier Transformation: Converts time-domain data into frequency-domain spectra, revealing chemical shifts and coupling patterns.
- Post-Processing Tools: Sophisticated algorithms are used to perform baseline correction, peak deconvolution, phase adjustment, and noise reduction. These techniques are essential for ensuring that even subtle spectral features are accurately resolved and quantified.
- Automation and AI Integration: Newer software platforms include automated peak picking and structure elucidation tools that reduce user error and streamline data interpretation.
Ways NMR Enhances Chemical Analysis Accuracy
Direct Structural Elucidation
NMR's greatest asset is its ability to directly elucidate molecular structure. Multidimensional NMR techniques such as COSY (Correlated Spectroscopy), HSQC (Heteronuclear Single Quantum Coherence), and HMBC (Heteronuclear Multiple Bond Correlation) provide two- and three-dimensional spectra that map proton-proton and proton-carbon interactions. This allows unambiguous determination of molecular backbones.
Quantitative Analysis
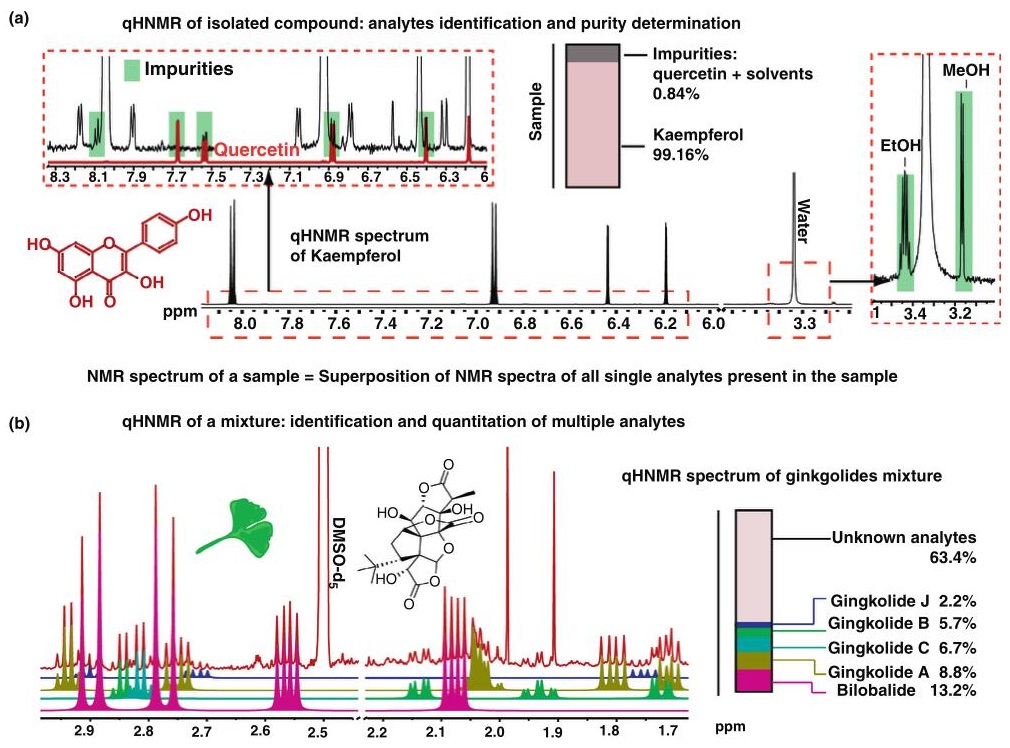
Quantitative NMR (qNMR) is a powerful technique that provides highly accurate concentration measurements without the need for calibration curves. By integrating signal areas and referencing to an internal standard, qNMR provides absolute quantification with unmatched precision—often within ±1-2%.
→ Case 1: Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay: mini perspective
Analysis of Complex Mixtures
One of the revolutionary features of NMR is its ability to analyze complex mixtures without prior separation. This is particularly valuable in natural products.
→ Case 2: Potential of in vitro nuclear magnetic resonance of biofluids and tissues in clinical research
Non-Destructive Testing
NMR is a non-destructive technique that allows repeated analysis of the same sample under different conditions. This feature increases reproducibility and allows longitudinal studies.
→ Case 3: Application and methodology of the non-destructive 19F time-domain NMR technique to measure the content in fluorine-containing drug products
Stereochemical and Chiral Analysis
NMR excels at stereochemical determination, especially when combined with chiral shift reagents. Techniques such as NOESY (Nuclear Overhauser Effect Spectroscopy) reveal the spatial proximity between atoms, which is invaluable in assigning stereochemistry.
→ Case 4: Applications of 31P NMR spectroscopy in development of M(Duphos)-catalyzed asymmetric synthesis of P-stereogenic phosphines (M=Pt or Pd)
Real-Time Reaction Monitoring
Using NMR, chemists can monitor reactions in real time. time-resolved NMR captures intermediate species, reaction kinetics, and product formation-all without quenching the reaction.
→ Case 5: Real-time reaction monitoring with in operando flow NMR and FTIR spectroscopy: reaction mechanism of benzoxazole synthesis
High Specificity for Elemental Nuclei
NMR is not limited to hydrogen and carbon. Multinuclear NMR extends the scope to elements such as phosphorus, fluorine, and nitrogen—critical for organometallics, coordination compounds, and drug formulations.
Applications Across Scientific Domains
Pharmaceutical Industry
In pharmaceutical research and development, NMR spectroscopy plays a key role at every stage—from drug discovery to final quality assurance. It is routinely used for:
- Structure Determination of Active Pharmaceutical Ingredients (APIs): NMR provides accurate 1D and 2D structural elucidation of small molecule drugs. It can confirm the identity and stereochemistry of APIs with high confidence, critical for regulatory approval and intellectual property protection.
- Purity Assessment and Quantification: NMR provides absolute quantification without the need for reference standards. Impurities and residual solvents can be detected with excellent sensitivity, ensuring drug safety and compliance with pharmacopeial standards.
- Polymorphism and Salt Form Studies: Solid-state NMR helps distinguish between polymorphic forms and different salt derivatives of a drug—variations that can drastically affect bioavailability and stability.
- Stability and Degradation Profiling: NMR monitors drug degradation pathways under stress conditions (e.g., heat, humidity, light). This information is critical for determining shelf life and establishing formulation strategies.
Materials Science
NMR spectroscopy is an increasingly important tool in materials science, especially for the characterization of crystalline and amorphous materials at the atomic level.
- Polymer Characterization: NMR determines molecular weight distribution, branching, tacticity, and cross-linking in synthetic and natural polymers. Solid-state NMR also studies the mobility and phase behavior of polymeric components.
- Nanomaterial Surface Chemistry: The surface functionalization of nanoparticles, quantum dots, and carbon-based nanostructures can be studied by NMR, often using dynamic nuclear polarization (DNP) techniques to enhance sensitivity.
- Porous Material Structure and Dynamics: In materials such as zeolites, metal-organic frameworks (MOFs), and silica gels, NMR reveals pore size, guest-host interactions, and local ordering - critical data for catalysis, separation, and gas storage applications.
Food and Nutritional Sciences
In food chemistry, NMR is used to ensure the authenticity, quality and nutritional content of food products, often as part of regulatory or industrial quality control workflows.
- Authentication and Adulteration Detection: NMR fingerprinting identifies food fraud—such as dilution, substitution or mislabeling of origin—in products such as olive oil, wine, honey and milk.
- Analysis of Antioxidants, Lipids, and Flavors: The composition and dynamics of bioactive food components can be studied in situ, allowing a better understanding of flavor release, shelf stability, and nutritional value.
- Quality Control of Nutraceuticals: For dietary supplements and functional foods, NMR verifies label claims by quantifying active ingredients such as polyphenols, amino acids and vitamins - ensuring both efficacy and safety.
Biomedical and Metabolomics
NMR is a cornerstone technique in metabolomics and systems biology due to its reproducibility, quantitative capabilities, and ability to analyze biofluids with minimal preparation.
- Metabolic Profiling of Biofluids: Urine, plasma, cerebrospinal fluid, and saliva can be profiled using NMR to reveal systemic metabolic changes associated with disease, diet, or drug exposure.
- Disease Biomarker Discovery: Statistical analysis of NMR metabolomics data (using PCA, OPLS-DA, etc.) helps identify potential biomarkers for diseases such as cancer, diabetes, cardiovascular conditions, and neurodegeneration.
- Personalized Medicine Studies: NMR can monitor individual metabolic responses to therapies, diet, and lifestyle, contributing to more tailored treatment strategies based on metabolic phenotyping.
Natural Products and Plant Metabolites
Natural product chemistry relies heavily on NMR for deciphering the complex architectures of secondary metabolites, which often contain multiple stereocenters and intricate ring systems.
- Dereplication of Known Compounds: NMR enables rapid identification of previously reported compounds in natural extracts, helping to avoid redundant isolation efforts and focus resources on novel structures.
- Structural Elucidation of Novel Bioactives: 2D NMR techniques such as HSQC, HMBC, and COSY are essential tools for piecing together the carbon-hydrogen framework of new natural products. Chiral NMR techniques also help to determine enantiomeric purity and absolute configuration.
- Chemotaxonomic Studies: NMR-based metabolite profiling is used to classify plant species based on their secondary metabolite content, providing insights into phylogenetic relationships and ecological functions.
Environmental Chemistry
NMR spectroscopy provides a powerful, non-invasive approach to analyzing the complex mixtures commonly found in environmental samples.
- Monitoring Pollutants in Water and Soil: NMR detects and quantifies organic contaminants, including persistent organic pollutants (POPs) and endocrine disruptors, with minimal sample preparation.
- Structural Identification of Complex Contaminants: Contaminants from industrial runoff, pharmaceuticals, or agricultural waste can be structurally characterized, providing clues to their sources and degradation pathways.
- Analysis of Microplastic Degradation Products: As microplastics degrade, NMR can monitor the formation of oligomers, additives, and oxidized by-products - providing insight into environmental toxicity and long-term fate.
Key Factors that Enhance Analytical Accuracy in NMR
- Resolution and Line Shape: High-resolution spectra allow better separation of overlapping peaks. Line broadening due to magnetic field inhomogeneity or sample issues can reduce accuracy.
- Sensitivity: Signal-to-noise ratio (SNR) defines how well peaks can be detected above baseline noise. Cryoprobes, high field magnets, and isotope enrichment increase sensitivity.
- Reproducibility: Modern NMR instruments provide exceptional reproducibility. Automatic sample changers, consistent shimming, and standardized pulse sequences help maintain consistent results from batch to batch.
- Calibration and Referencing: Chemical shifts are referenced to known standards such as TMS (tetramethylsilane) for ¹H and ¹³C. Regular calibration minimizes drift and systemic error.
Case Studies
Case 1: Importance of purity evaluation and the potential of quantitative 1H NMR as a purity assay: mini perspective
In biomedical and chemical research, both structure and purity are essential to accurately describe a compound, especially in drug development. Quantitative NMR (qNMR)—in particular proton NMR (qHNMR)—provides a powerful, universal and orthogonal method for assessing purity, surpassing traditional techniques such as chromatography and elemental analysis. qHNMR can detect analytes that are often missed, such as water and sorbents, and can be easily adapted from routine structural NMR workflows.
The most common approach to absolute quantification in qHNMR is the addition of a well-characterized internal calibrator to the sample. Ideal calibrators should be pure, stable, inexpensive, non-toxic, and show no signal interference from the analyte. Dimethylsulfone (DMSO2) is highlighted as a particularly effective calibrator. The study provides a framework for standard qHNMR assays and includes detailed methodology and calculations to ensure accurate and precise purity assessments.
 Figure 5. Application of the internal calibration method for absolute qHNMR analysis. A commercial sample of (+)-catechin (5.33 mg/mL in DMSO-D6, 600 MHz; declared purity >96%) was analyzed. Dimethylsulfone (DMSO2, 99.4% pure) was added to the sample as internal calibrant using a stock solution (2.28 mg/mL; final concentration in the sample 0.380 mg/mL). The content of (+)-catechin in the sample was established as 98.2% w/w. (Pauli et al., 2014)
Figure 5. Application of the internal calibration method for absolute qHNMR analysis. A commercial sample of (+)-catechin (5.33 mg/mL in DMSO-D6, 600 MHz; declared purity >96%) was analyzed. Dimethylsulfone (DMSO2, 99.4% pure) was added to the sample as internal calibrant using a stock solution (2.28 mg/mL; final concentration in the sample 0.380 mg/mL). The content of (+)-catechin in the sample was established as 98.2% w/w. (Pauli et al., 2014)
Case 2: Potential of in vitro nuclear magnetic resonance of biofluids and tissues in clinical research
Body fluids, cells, and tissues contain complex mixtures of low-molecular-weight metabolites, making their comprehensive analysis a challenge. Quantitative NMR spectroscopy has emerged as a powerful technique in metabolomics due to its speed, reproducibility, minimal sample preparation, and ability to quantify a wide range of endogenous metabolites. NMR-based metabolomics is increasingly being applied in various medical fields such as microbiology, toxicology, pharmacology, and diagnostics. This review highlights its clinical utility, emphasizing in vitro applications for detecting metabolic changes and identifying potential biomarkers for early disease diagnosis and prognosis.
 Figure 6. One-dimensional (1D) proton nuclear magnetic resonance (1H NMR) spectra (aliphatic region) acquired at 700 MHz in D2Oat 25Cof(A) Urine, (B) Blood plasma, and (C) Perchloric acid extract of intestinal mucosal biopsy obtained from a patient with celiac disease. Several metabolites, such as amino acids, lipids, sugars, and organic acids, were observed in the spectra. (Dubey et al., 2023)
Figure 6. One-dimensional (1D) proton nuclear magnetic resonance (1H NMR) spectra (aliphatic region) acquired at 700 MHz in D2Oat 25Cof(A) Urine, (B) Blood plasma, and (C) Perchloric acid extract of intestinal mucosal biopsy obtained from a patient with celiac disease. Several metabolites, such as amino acids, lipids, sugars, and organic acids, were observed in the spectra. (Dubey et al., 2023)
Case 3: Application and methodology of the non-destructive ¹⁹F time-domain NMR technique to measure the content in fluorine-containing drug products
The study presents a low-field fluorine-19 (¹⁹F) time-domain NMR protocol for quantifying fluorinated drug content directly in tablets or capsules. This technique is specific to fluorinated drugs and avoids interference from non-fluorinated excipients. Compared to high-field NMR, the method is simpler, less expensive, non-destructive and suitable for routine use. It has been successfully tested on commercial drugs such as cinacalcet, lansoprazole and ciprofloxacin, confirming label accuracy. The protocol's reliability and affordability make it ideal for wider use, including as a process analytical technology (PAT) in pharmaceutical manufacturing.
 Figure 7. NMR Data. NMR signal as a function of percentage of drug substance as free base for intact and crushed tablets of ciprofloxacin (blue dots) when using (A) ciprofloxacin HCl monohydrate (red dots) and (B) ciprofloxacin free base (red dots) as reference standards. The data was generated using Mnova software. (Silva et al., 2017)
Figure 7. NMR Data. NMR signal as a function of percentage of drug substance as free base for intact and crushed tablets of ciprofloxacin (blue dots) when using (A) ciprofloxacin HCl monohydrate (red dots) and (B) ciprofloxacin free base (red dots) as reference standards. The data was generated using Mnova software. (Silva et al., 2017)
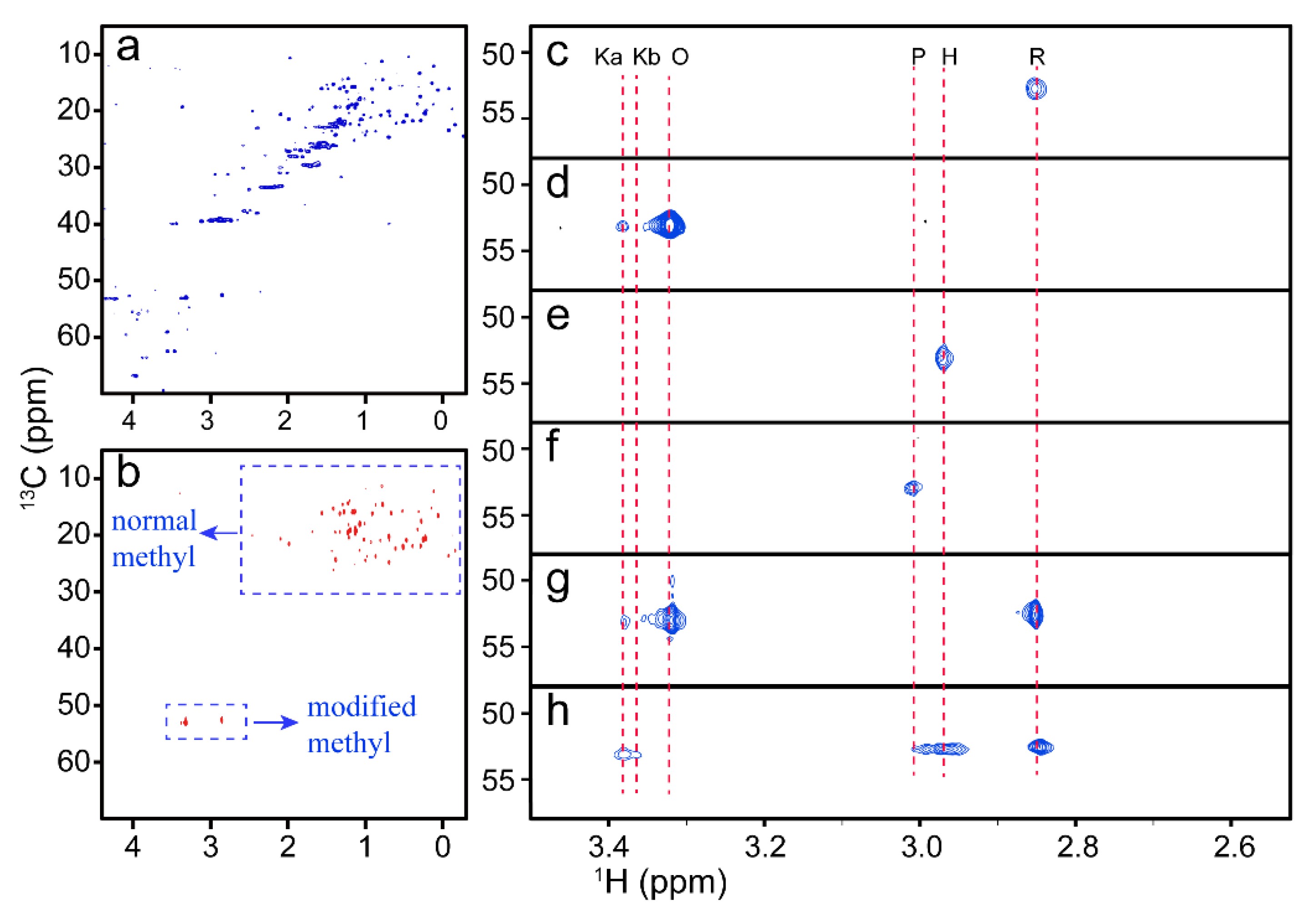
Case 4: Applications of ³¹P NMR spectroscopy in development of M(Duphos)-catalyzed asymmetric synthesis of P-stereogenic phosphines (M=Pt or Pd)
This study highlights the application of ³¹P NMR spectroscopy in an ongoing research project focused on the enantioselective P-C bond formation catalyzed by platinum and palladium duphos complexes. The technique is used to characterize catalyst precursors, intermediates and final products, and to determine equilibrium and rate constants. In addition, it allows the measurement of the enantiomeric excess (ee) of P-stereogenic phosphine products. The discussion also covers how ³¹P NMR can aid in problem solving, identification of unexpected reaction products, and interpretation of a particularly distinctive and visually striking spectrum.
 Figure 8. 202 MHz ³¹P¹H NMR spectrum (CD2Cl2) of the complexes [Pd(S-C10H6CHMeNMe2)(Cl)]2(μ-Ph(CH2Ph)PCH2CH2PPh(CH2Ph), containing diastereomerically and enantiomerically enriched diphosphines prepared with Pt(Duphos) catalysts of different absolute configuration. (Glueck, 2008)
Figure 8. 202 MHz ³¹P¹H NMR spectrum (CD2Cl2) of the complexes [Pd(S-C10H6CHMeNMe2)(Cl)]2(μ-Ph(CH2Ph)PCH2CH2PPh(CH2Ph), containing diastereomerically and enantiomerically enriched diphosphines prepared with Pt(Duphos) catalysts of different absolute configuration. (Glueck, 2008)
Case 5: Real-time reaction monitoring with in operando flow NMR and FTIR spectroscopy: reaction mechanism of benzoxazole synthesis
This study presents a combined flow FTIR and NMR spectroscopy system designed for in operando monitoring of reaction intermediates to better understand reaction mechanisms. By integrating a flow cell with an FTIR spectrometer, the setup enhances sensitivity to weak IR signals, while coupled flow NMR enables quantitative analysis of reaction species. This dual-system approach overcomes limitations of IR spectroscopy, such as varying absorption coefficients, and successfully elucidated the controversial mechanism of benzoxazole synthesis. The setup also provided accurate kinetic data, including reaction timing and rate-determining steps. Overall, this technique provides a powerful and safe method for real-time analysis of complex or hazardous chemical reactions.
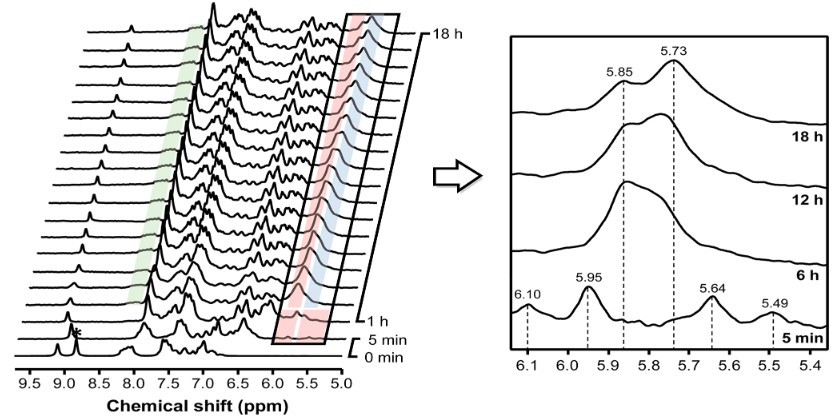 Figure 9. In operando flow 1H NMR (60 MHz) reaction profile. (Chae et al., 2021)
Figure 9. In operando flow 1H NMR (60 MHz) reaction profile. (Chae et al., 2021)
Experience unparalleled accuracy in your chemical research with Creative Biostructure's advanced NMR services. Whether you're analyzing complex compounds or fine-tuning molecular structures, we have the tools and expertise to ensure accurate, reproducible results. Contact us today to learn how our NMR solutions can elevate the accuracy of your analysis and accelerate your discoveries!
References
- Chae Y, Min S, Park E, et al. Real-time reaction monitoring with in operando flow NMR and FTIR spectroscopy: reaction mechanism of benzoxazole synthesis. Anal Chem. 2021;93(4):2106-2113. doi:10.1021/acs.analchem.0c03852
- Dubey R, Sinha N, Jagannathan NR. Potential of in vitro nuclear magnetic resonance of biofluids and tissues in clinical research. NMR in Biomedicine. 2023;36(4):e4686. doi:10.1002/nbm.4686
- Gebretsadik T, Linert W, Thomas M, Berhanu T, Frew R. LC–NMR for natural product analysis: a journey from an academic curiosity to a robust analytical tool. Sci. 2021;3(1):6. doi:10.3390/sci3010006
- Glueck DS. Applications of 31P NMR spectroscopy in development of M(Duphos)-catalyzed asymmetric synthesis of P-stereogenic phosphines (M=Pt or Pd). Coordination Chemistry Reviews. 2008;252(21-22):2171-2179. doi:10.1016/j.ccr.2007.12.023
- Han R, Wang S. Mechanisms of antimicrobial peptides as characterized by solid-state NMR. Magnetic Resonance Letters. 2022;2(2):119-129. doi:10.1016/j.mrl.2021.09.004
- Liu Y, Gao L, Yu Z. Quantitative 31P NMR spectroscopy: principles, methodologies, and applications in phosphorus-containing compound analysis. Applied Sciences. 2024;15(1):323. doi:10.3390/app15010323
- Pauli GF, Chen SN, Simmler C, et al. Importance of purity evaluation and the potential of quantitative1 h nmr as a purity assay: mini perspective. J Med Chem. 2014;57(22):9220-9231. doi:10.1021/jm500734a
- Sannigrahi P, Ingall E. Polyphosphates as a source of enhanced P fluxes in marine sediments overlain by anoxic waters: Evidence from 31P NMR. Geochem Trans. 2005;6(3):52. doi:10.1186/1467-4866-6-52
- Silva Elipe MV, Li L, Nagapudi K, et al. Application and methodology of the non-destructive 19F time-domain NMR technique to measure the content in fluorine-containing drug products. JoVE. 2017;(126):55850. doi:10.3791/55850