Glycomics—the comprehensive study of the structure, function, and biology of carbohydrates and glycoconjugates—is a rapidly expanding discipline in systems biology, medicine, biotechnology, and food science. Carbohydrates are key players in cell signaling, molecular recognition, and structural integrity. However, their complex branching, stereochemical diversity, and conformational flexibility make them analytically challenging.
Among analytical methods, Nuclear Magnetic Resonance (NMR) spectroscopy offers unique advantages for sugar analysis: it is non-destructive, provides detailed structural information, and is capable of studying molecules in solution or in situ. Unlike mass spectrometry (MS), which excels at sensitivity, NMR provides precise information on atomic connectivity, linkage positions, and stereochemistry.
Creative Biostructure offers advanced NMR services tailored to provide comprehensive solutions. In this article, we present an in-depth overview of NMR applications in glycomics and sugar analysis, covering both methodological insights and practical examples to support professionals across various disciplines.
 Figure 1. An example for functional glycomics: Cell-cell interactions are mediated through glycan-protein binding. (Zaia, 2011)
Figure 1. An example for functional glycomics: Cell-cell interactions are mediated through glycan-protein binding. (Zaia, 2011)
The Unique Challenges of Glycomics
Carbohydrates exhibit several characteristics that complicate their structural analysis:
- High Stereochemical Complexity: Even small oligosaccharides can have multiple stereoisomers due to different anomeric configurations and sugar ring types (pyranose vs. furanose).
- Branched Structures: Glycans often display complex branching, in contrast to the linearity of nucleic acids or proteins.
- Conformational Flexibility: Sugars can adopt multiple conformers, complicating spectral interpretation.
- Isomerism: Monosaccharides may differ only in the configuration of a single hydroxyl group or linkage site, but this can drastically affect biological function.
- Lack of Chromophores: This limits UV-based detection, further increasing the importance of NMR.
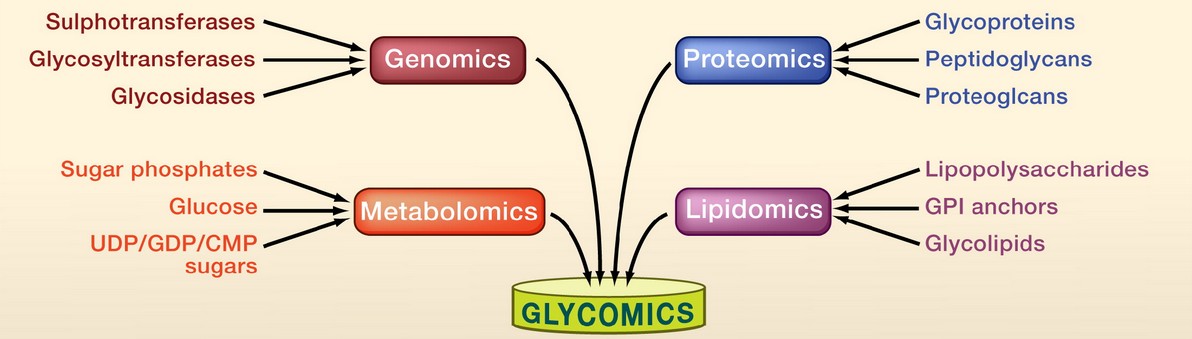 Figure 2. Glycomic complexity. (Adapted from Hart and Copeland, 2010)
Figure 2. Glycomic complexity. (Adapted from Hart and Copeland, 2010)
NMR meets these challenges by providing atomic-level resolution without requiring derivatization or labeling, allowing for direct observation of glycans in their native states.
Structural Features of Carbohydrates Explored by NMR
Carbohydrates are complex biomolecules with a variety of structural features that can be effectively resolved using NMR spectroscopy. These features include:
Ring Forms (Pyranose vs. Furanose)
Carbohydrates can exist in different ring forms, such as pyranose (six-membered ring) and furanose (five-membered ring). NMR can distinguish between these forms based on the characteristic chemical shifts of the protons and carbons in the ring structure. For example, pyranose forms typically exhibit distinct chemical shifts compared to furanose forms, allowing researchers to identify the specific ring conformation present in a carbohydrate.
Anomeric Configuration (α vs. β)
The anomeric configuration of carbohydrates, which refers to the stereochemistry at the anomeric carbon (α or β), is crucial for understanding their reactivity and biological function. NMR can readily differentiate between α- and β-anomers by analyzing the chemical shifts of the anomeric proton and carbon. The coupling constants between the anomeric proton and adjacent protons can also provide additional information about the configuration.
 Figure 3. 19F-1H NMR spectrum of FDGal-6 pyranose anomers in D2O, 565 MHz. (Adapted from Poškaitė et al., 2023)
Figure 3. 19F-1H NMR spectrum of FDGal-6 pyranose anomers in D2O, 565 MHz. (Adapted from Poškaitė et al., 2023)
Inter-glycosidic Linkages (e.g., 1→4, 1→6)
NMR is highly effective in identifying the types of glycosidic linkages in oligo- and polysaccharides. By examining the coupling constants and chemical shifts of the protons involved in the glycosidic bonds, researchers can determine the specific linkages present, such as 1→4 or 1→6 linkages. This information is essential for understanding the structure and function of complex carbohydrate chains.
Branching Patterns
Carbohydrates often exhibit branching, which can significantly influence their properties and interactions. NMR can reveal branching patterns by analyzing the chemical shifts and coupling constants of the protons and carbons at the branching points. This allows researchers to map out the overall structure of branched oligo- and polysaccharides, even in complex mixtures.
Hydrogen-Bond Networks
Hydrogen bonding plays a critical role in the stability and function of carbohydrates. NMR can detect hydrogen-bond networks by observing changes in chemical shifts and coupling constants of the involved protons. For example, hydrogen-bonded protons often exhibit downfield shifts, providing valuable insights into the hydrogen-bonding interactions within carbohydrate structures.
Substituent Effects (e.g., sulfation, acetylation, methylation)
Carbohydrates can be modified by various substituents, such as sulfate, acetate, or methyl groups, which can significantly affect their chemical and biological properties. NMR can identify and characterize these modifications by analyzing the changes in chemical shifts and coupling constants of the protons and carbons adjacent to the substituents. This allows researchers to determine the exact location and type of substitution in the carbohydrate structure.
Quantitative NMR (qNMR) for Sugar Analysis
Quantitative NMR (qNMR) is a powerful technique for the absolute quantification of sugars, eliminating the need for external standards. This method relies on the inherent quantitative nature of NMR, where the signal intensity directly correlates with the concentration of the analyte. qNMR is particularly useful for:
- Determining sugar content in food matrices: qNMR can accurately quantify sugars in complex food samples such as honey, fruit juices, and other beverages. This is crucial for quality control and nutritional labeling.
- Monitoring enzymatic digestion of polysaccharides: qNMR allows real-time tracking of the breakdown of complex carbohydrates by enzymes, providing insights into digestion kinetics and mechanisms.
- Quantifying monosaccharides in hydrolysates: qNMR is used to measure the concentration of individual monosaccharides in hydrolyzed samples, which is essential for studying carbohydrate metabolism and bioconversion processes.
NMR in Metabolomics and Clinical Glycomics
Glycomics, the study of glycans, intersects with metabolomics to investigate physiological and pathological processes. NMR plays a key role in this field by:
- Profiling urinary or plasma sugars in metabolic diseases: NMR can identify and quantify sugars in biofluids, providing biomarkers for conditions such as diabetes and metabolic syndrome.
- Monitoring gut microbiota metabolism of dietary polysaccharides: NMR helps track how gut bacteria metabolize complex carbohydrates, influencing gut health and overall metabolism.
- Detecting glycan-based biomarkers in cancer and inflammation: NMR can identify specific glycan signatures associated with diseases, aiding in early diagnosis and treatment monitoring.
1H-NMR metabolomics workflows, combined with multivariate statistical analyses like Principal Component Analysis (PCA) and Orthogonal Projection to Latent Structures (OPLS), correlate glycan profiles with disease states, offering valuable diagnostic tools.
NMR for Studying Glycan-Protein Interactions
Understanding glycan-protein interactions is critical for drug development, immunology, and infection biology. Key NMR techniques include:
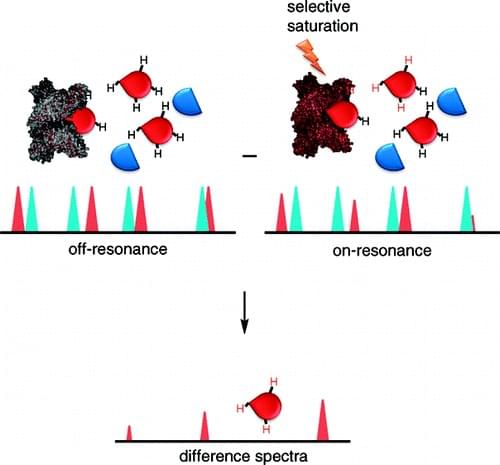
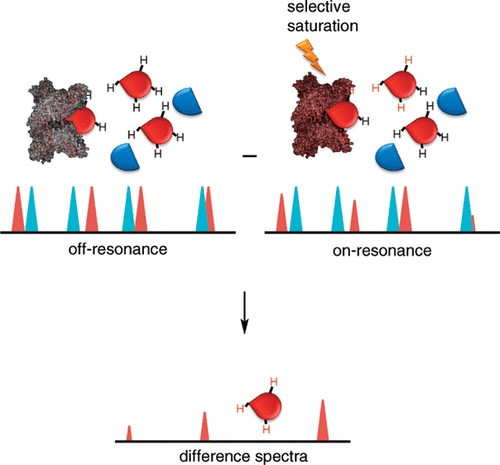
- STD-NMR (Saturation Transfer Difference): Maps glycan epitopes that bind to protein surfaces, identifying specific interaction sites.
- WaterLOGSY: Detects weak ligand binding through solvent-exchange behavior, useful for identifying low-affinity interactions.
- Transferred NOE (trNOE): Determines ligand conformation when bound to a protein, providing structural insights.
- Chemical shift perturbation: Indicates residues involved in binding, revealing interaction hotspots.
For example, STD-NMR has been used to identify specific sugar residues in sialylated oligosaccharides that bind to influenza hemagglutinin, aiding in the development of antiviral strategies.
Integration with Other Analytical Techniques
While NMR provides unparalleled structural detail, it is often integrated with other techniques to enhance glycan characterization:
- Mass Spectrometry (MS): Offers high sensitivity and mass accuracy, ideal for detecting low-abundance glycans.
- High-Performance Anion Exchange Chromatography (HPAEC-PAD): Provides detailed monosaccharide profiling, complementing NMR's structural information.
- Capillary Electrophoresis (CE): Separates isomers and charge variants, useful for resolving complex glycan mixtures.
- X-ray crystallography: Validates structures, particularly for glycoproteins, providing detailed 3D information.
This integration enables comprehensive glycan characterization, from composition and structure to dynamics and interactions.
Select Service
- Quantitative NMR
- Food Analysis
- NMR Analysis of Food Adulteration
- Qualitative Analysis Food Polysaccharides by NMR
- Metabolic Profiling for Molecular Complexity
- Saturation Transfer Differences (STD) NMR
- Pure Shift NMR Service
- Combining NMR and Mass Spectrometry for Metabolomics
- Custom Ion-Exchange Chromatography Service
- NMR Crystallography Service
- X-ray Crystallography Services
Case Studies
Case 1: Solution NMR for carbohydrate structure via hydroxyl mapping
This study presents an efficient NMR-based strategy for elucidating the chemical structures of glucose and four glucose-containing disaccharides—nigerose, gentiobiose, leucrose, and isomaltulose. The method begins by analyzing the 1H resonances of hydroxyl (−OH) groups, observable in the NMR spectrum of a supercooled aqueous solution, and then applies the 2D HSQC-TOCSY technique to determine the covalent topology of the molecules, including C–C, C–H, and O–H connectivity. This approach enables full 1H and 13C resonance assignment and is demonstrated using glucose as a model "unknown" compound.
The technique is particularly advantageous for characterizing novel or scarce carbohydrates and is proposed to be broadly applicable to larger, biologically derived sugars. By initiating structural analysis from the −OH resonances, the method parallels established peptide/protein NMR strategies that begin with −NH resonances, offering a logical and effective entry point into carbohydrate structure determination.
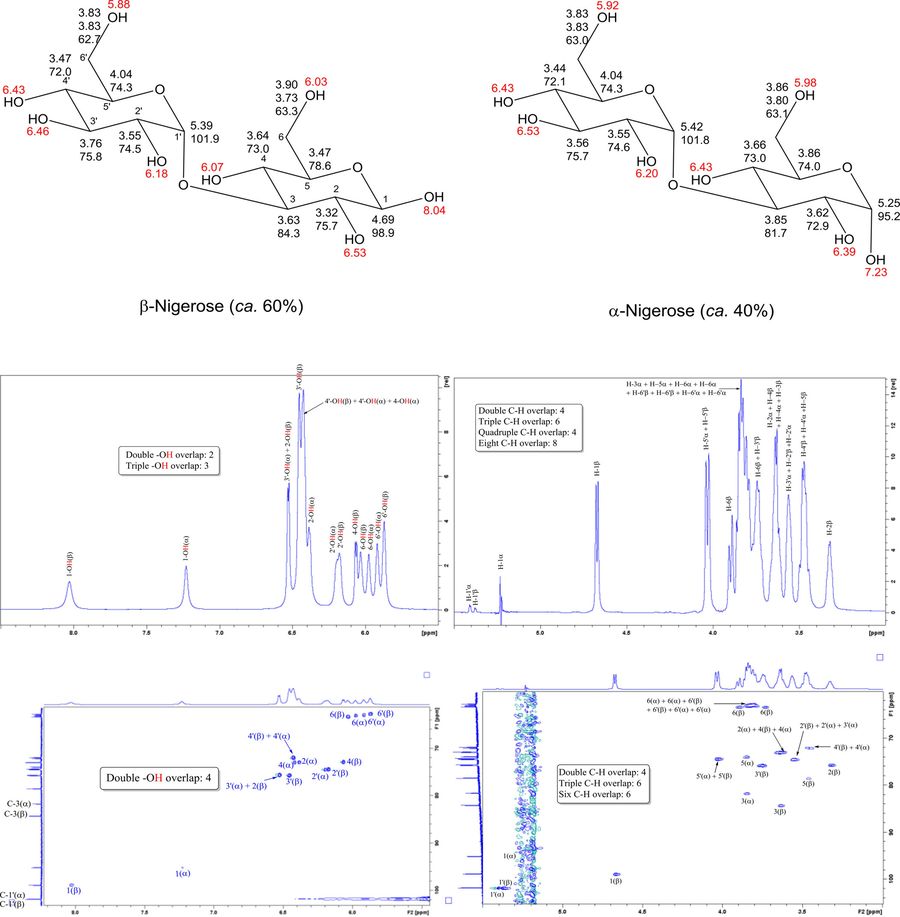 Figure 4. 1H NMR assignments of −OH resonances (red) and C–H resonances (bold) for supercooled solutions of the α- and β-anomers for nigerose (Glcα1-3Glc). 13C resonances that are shared by these two sets of protons are shown below the corresponding 1H assignments for the C–H groups. Assignments were deduced from 1D NMR (top left is an expansion of the −OH region and top right is an expansion of the C–H region from the 1H NMR spectrum) and 2D NMR. (Brown et al., 2018)
Figure 4. 1H NMR assignments of −OH resonances (red) and C–H resonances (bold) for supercooled solutions of the α- and β-anomers for nigerose (Glcα1-3Glc). 13C resonances that are shared by these two sets of protons are shown below the corresponding 1H assignments for the C–H groups. Assignments were deduced from 1D NMR (top left is an expansion of the −OH region and top right is an expansion of the C–H region from the 1H NMR spectrum) and 2D NMR. (Brown et al., 2018)
Case 2: NMR insights into glycan–lectin interactions in native-like environments
This study highlights the application of diverse NMR methodologies to investigate glycan–lectin interactions under conditions that closely mimic the native cellular environment. While traditional NMR approaches typically study isolated glycans and lectins in solution, this work expands the scope by incorporating both receptor- and ligand-based techniques to observe molecular recognition events in more biologically relevant settings. Using galectin-7 as a model, the study reports the first intracellular observation of a folded lectin's 1H-15N HMQC NMR spectrum in Danio rerio oocytes, facilitated by a glycomimetic ligand (TDG) that minimizes nonspecific binding.
In parallel, ligand-based STD-NMR experiments were employed to study interactions between Siglec-10 and various glycans or glycomimetics presented on the cell surface. These findings demonstrate that NMR can effectively capture glycan–lectin recognition in complex biological contexts, whether inside cells or on the cell membrane. Together, the methodologies presented provide a foundation for future NMR-based studies of glycan interactions in native-like environments, advancing our understanding of glycan-mediated signaling and recognition.
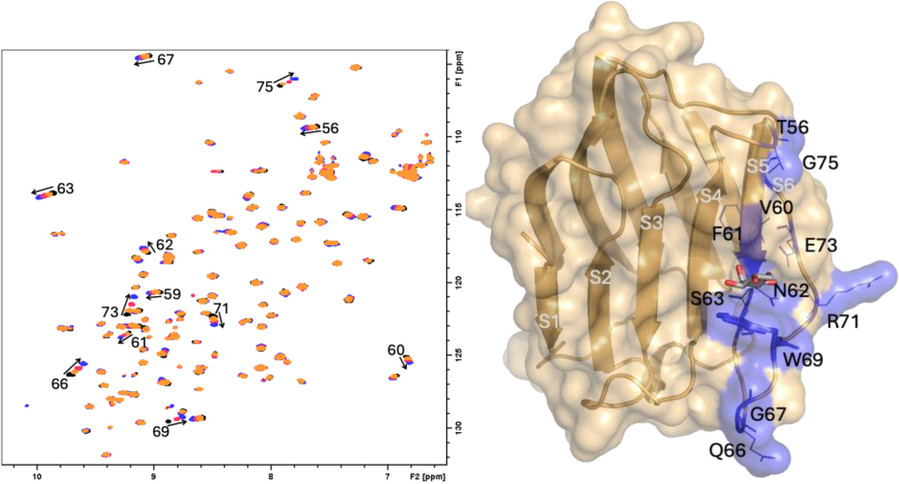 Figure 5. The interaction between Gal7 and TDG by 1H,15N-HSQC-NMR. On the left: superimposition of 1H, 15N-HSQC spectra of 15N-Gal7 upon increasing additions of TDG: black, no TDG; orange, 1.5 eq TDG; red 5 eq TDG and blue 60 eq TDG. On the right: 3D structure of Gal7 (PDB 4xbq). Amino acids annotated in the spectra on the left are highlighted in blue and represents as thin lines. (Bertuzzi et al., 2025)
Figure 5. The interaction between Gal7 and TDG by 1H,15N-HSQC-NMR. On the left: superimposition of 1H, 15N-HSQC spectra of 15N-Gal7 upon increasing additions of TDG: black, no TDG; orange, 1.5 eq TDG; red 5 eq TDG and blue 60 eq TDG. On the right: 3D structure of Gal7 (PDB 4xbq). Amino acids annotated in the spectra on the left are highlighted in blue and represents as thin lines. (Bertuzzi et al., 2025)
In summary, NMR spectroscopy is an indispensable tool for glycomics, offering rich structural, quantitative, and dynamic data. Its capacity to elucidate linkage patterns, anomeric configurations, branching architectures, and conformations makes it essential for carbohydrate chemistry and biology. Despite certain limitations in sensitivity and sample requirements, advances in instrumentation, pulse sequence development, and data analytics continue to expand the power of NMR in sugar research. When combined with orthogonal methods like MS and crystallography, NMR provides a complete toolkit for modern glycan science—from fundamental studies to applications in health, food, and biotechnology.
At Creative Biostructure, we specialize in advanced NMR services for sugar and glycan analysis, including 1D/2D NMR, dynamics studies, and in-cell NMR techniques. From structural elucidation to biomolecular interaction mapping, our customized solutions are designed to meet the unique challenges of glycoscience. Contact us today to discuss how our NMR expertise can support your next glycomics breakthrough!
References
- Bertuzzi S, Lete MG, Franconetti A, et al. Exploring glycan‐lectin interactions in natural‐like environments: a view using NMR experiments inside cell and on cell surface. Chemistry A European J. 2025;31(10):e202403102.
- Brown GD, Bauer J, Osborn HMI, Kuemmerle R. A solution NMR approach to determine the chemical structures of carbohydrates using the hydroxyl groups as starting points. ACS Omega. 2018;3(12):17957-17975.
- Hart GW, Copeland RJ. Glycomics hits the big time. Cell. 2010;143(5):672-676.
- Poškaitė G, Wheatley DE, Wells N, Linclau B, Sinnaeve D. Obtaining pure 1H NMR spectra of individual pyranose and furanose anomers of reducing deoxyfluorinated sugars. J Org Chem. 2023;88(19):13908-13925.
- Zaia J. At last, functional glycomics. Nat Methods. 2011;8(1):55-57.