Metabolomics, the comprehensive study of small-molecule metabolites within a biological system, plays a crucial role in systems biology, clinical diagnostics, and drug development. It aims to capture dynamic metabolic responses to various physiological stimuli, disease states, or therapeutic interventions. Among the arsenal of analytical techniques available for metabolomics, Nuclear Magnetic Resonance (NMR) spectroscopy stands out as a non-destructive, reproducible, and quantitative approach. NMR spectroscopy provides unique insights into metabolite identity, concentration, and structural information without requiring extensive sample preparation or chemical derivatization.
At Creative Biostructure, we specialize in advanced analytical solutions for metabolomics, including sample preparation and data interpretation. Our NMR-based platforms are designed to streamline biomarker discovery with high reproducibility, robust quantitation, and minimal sample handling, making us a trusted partner in academic and industrial settings. In this article, we will explore the application of NMR spectroscopy in metabolomics, emphasizing its principles, methodologies, strengths, limitations, and specific role in biomarker discovery.
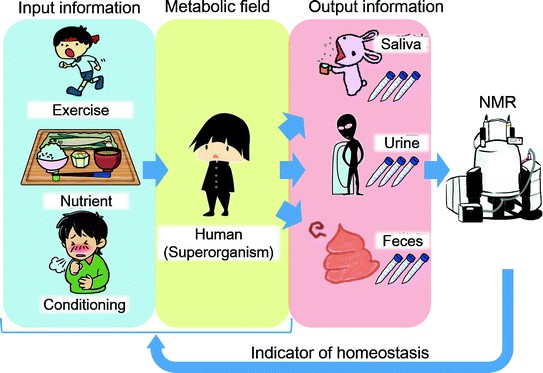
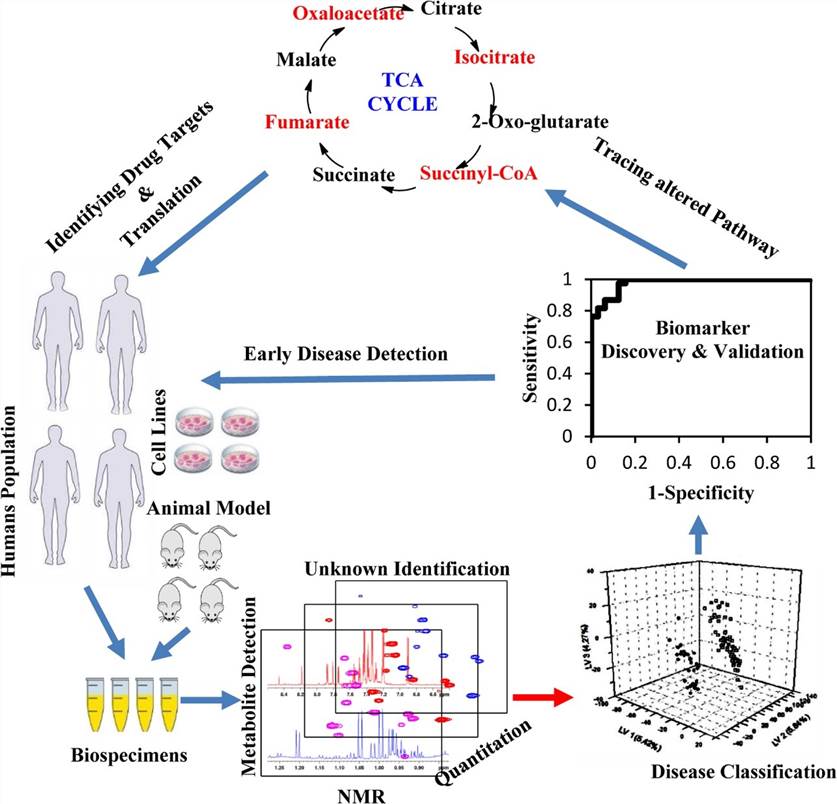
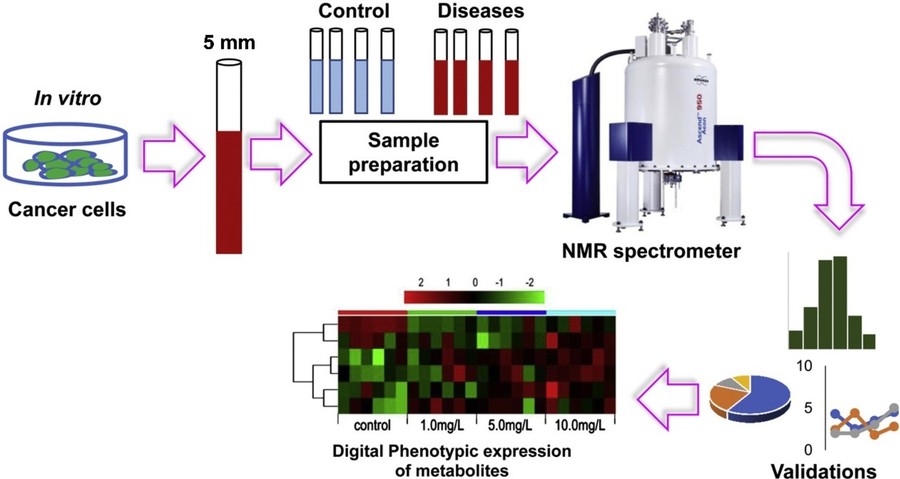 Figure 1. An example of NMR in metabolomics for biomarker discovery: 1H NMR-based metabolomics for cancer targeting and metabolic engineering. (Raja et al., 2020)
Figure 1. An example of NMR in metabolomics for biomarker discovery: 1H NMR-based metabolomics for cancer targeting and metabolic engineering. (Raja et al., 2020)
Principles of NMR Spectroscopy
NMR spectroscopy is based on the magnetic properties of certain atomic nuclei. Nuclei such as 1H, 13C, 15N, and 31P possess a magnetic moment due to their nuclear spin. When placed in an external magnetic field, these nuclei align with or against the field, creating distinct energy states. Upon exposure to a radiofrequency (RF) pulse, the nuclei absorb energy and transition between these states. The emitted signals as they relax back to equilibrium are detected and translated into NMR spectra.
The resonance frequency of each nucleus depends on its electronic environment, giving rise to a chemical shift. This chemical shift is a critical parameter for structural elucidation. Coupling constants, line widths, relaxation times (T1 and T2), and signal intensities provide additional layers of molecular information.
NMR in Metabolomics: Advantages and Challenges
NMR offers several compelling advantages in metabolomics:
- Non-Destructive Analysis: Samples remain intact and can be reused or analyzed using complementary techniques.
- Minimal Sample Preparation: No need for derivatization or separation.
- Quantitative Data: Signal intensities correlate directly with molar concentrations.
- High Reproducibility: Excellent for longitudinal and multi-center studies.
- Structural Insights: Capable of identifying unknown metabolites through spectral interpretation.
- Versatility: Applicable to biofluids (e.g., plasma, urine), tissues, and cell extracts.
However, some challenges remain:
- Sensitivity Limitations: NMR is less sensitive than mass spectrometry (MS), requiring higher metabolite concentrations.
- Spectral Overlap: Particularly in 1D spectra of complex mixtures.
- Instrumentation Cost: High-field NMR instruments involve substantial investment and maintenance.
NMR Techniques in Metabolomics
One-Dimensional NMR
- 1H NMR (Proton NMR): Proton NMR is the most widely used technique in metabolomics due to the natural abundance of hydrogen atoms in most biological metabolites. It provides rapid spectral acquisition, excellent reproducibility, and broad coverage of metabolite classes such as amino acids, sugars, organic acids, and lipids. Its minimal sample preparation requirements and non-destructive nature make it ideal for high-throughput and longitudinal studies, particularly when consistent analytical performance is required across multiple sample sets or experimental conditions.
- 13C and 31P NMR: 13C and 31P NMR are less sensitive than 1H NMR but offer valuable complementary information. 13C NMR is used to elucidate the carbon backbone of metabolites and is often employed with isotope-labeled substrates in metabolic flux analysis. Meanwhile, 31P NMR selectively detects phosphorylated compounds, making it particularly useful in studies of energy metabolism and phospholipid biochemistry. These nuclei expand the metabolomic window beyond what proton detection alone can provide, especially in targeted or pathway-specific investigations.
Two-Dimensional NMR
To overcome spectral crowding in complex samples, two-dimensional (2D) NMR techniques are employed:
- COSY (Correlation Spectroscopy): COSY is a homonuclear 2D NMR method that maps through-bond couplings between protons, allowing researchers to identify connected proton networks within a molecule. This is particularly useful for confirming the structure of known metabolites or characterizing novel ones in complex mixtures where peak overlap hinders one-dimensional interpretation.
- TOCSY (Total Correlation Spectroscopy): TOCSY extends the utility of COSY by identifying all coupled spins within a spin system, effectively connecting multiple proton environments across a molecular framework. This technique is instrumental for identifying complete substructures, such as carbohydrate rings or amino acid side chains, and is essential for deconvoluting spectral data in crowded biological samples.
- HSQC (Heteronuclear Single Quantum Coherence): HSQC correlates protons with directly bonded heteronuclei—most commonly 13C or 15N—thus enhancing spectral dispersion and facilitating the assignment of carbon–hydrogen or nitrogen–hydrogen pairs. Its sensitivity and resolution make it a cornerstone for accurate metabolite annotation, especially in isotope-labeled samples or complex biological matrices.
- HMBC (Heteronuclear Multiple Bond Correlation): HMBC detects long-range couplings between protons and heteronuclei across two or three bonds. It is particularly valuable for connecting distant parts of a molecule, enabling researchers to reconstruct carbon skeletons and determine connectivity in complex metabolites where direct correlations are insufficient.
Quantitative NMR (qNMR)
Quantitative NMR enables absolute metabolite quantification by comparing signal intensities to those of a known internal standard, such as TSP or DSS. Unlike many other techniques, qNMR does not require calibration curves, making it both time-efficient and highly reproducible. It is particularly useful in biomarker validation, pharmacokinetic modeling, nutritional assessments, and any application where accurate concentration measurements are critical. The method is inherently non-destructive and highly linear, lending itself well to repeated measures and longitudinal sampling in clinical studies.
High-Resolution Magic Angle Spinning (HR-MAS)
High-resolution magic angle spinning NMR is uniquely suited for analyzing semi-solid or intact biological samples such as tissues, biopsies, and whole cells. By spinning the sample at 54.7 degrees—the so-called magic angle—relative to the magnetic field, HR-MAS reduces line broadening due to anisotropic interactions, yielding high-resolution spectra from heterogeneous specimens. This technique allows researchers to assess metabolic profiles in situ without the need for extraction, thereby preserving spatial and biochemical integrity. HR-MAS has become increasingly important in clinical metabolomics, particularly for tumor tissue profiling and disease-specific metabolic fingerprinting.
 Figure 2. High-resolution magic angle spinning nuclear magnetic resonance spectroscopy of paired clinical liver tissue samples from hepatocellular cancer and surrounding region. (Fernandes et al., 2024)
Figure 2. High-resolution magic angle spinning nuclear magnetic resonance spectroscopy of paired clinical liver tissue samples from hepatocellular cancer and surrounding region. (Fernandes et al., 2024)
Hyperpolarization Techniques
- Dynamic Nuclear Polarization (DNP): DNP dramatically enhances NMR sensitivity by transferring polarization from unpaired electrons to nuclei under microwave irradiation. This results in signal enhancements of several orders of magnitude, enabling the detection of low-abundance metabolites and real-time monitoring of metabolic processes. DNP is especially powerful when studying metabolic flux using hyperpolarized 13C-labeled substrates, such as pyruvate, in both in vitro and in vivo systems.
- Parahydrogen-Induced Polarization (PHIP): PHIP employs para-hydrogen to chemically induce spin polarization during hydrogenation reactions. While less versatile than DNP, it offers a cost-effective approach to enhancing NMR signals, particularly in real-time studies of enzymatic transformations or rapid metabolic changes. PHIP has shown promise in expanding the practical applications of NMR to kinetic and mechanistic metabolomics.
Sample Preparation and Standardization
Proper sample preparation is critical for robust NMR-based metabolomics:
- Biofluids: Biofluids such as urine, serum, plasma, and cerebrospinal fluid are commonly analyzed in metabolomics due to their accessibility and rich metabolic content. Before NMR acquisition, samples typically undergo centrifugation to remove cells or particulates, followed by filtration to eliminate macromolecules that may obscure spectral resolution. A small volume of deuterium oxide (D2O) is added to each sample to enable field locking, which stabilizes the magnetic field during acquisition. Additionally, a buffer system-commonly phosphate or Tris-is used to maintain a consistent and physiologically relevant pH, as even slight pH variations can cause chemical shift changes and complicate spectral interpretation.
- Tissue Extracts: Metabolic profiling of tissues requires extraction protocols that preserve the integrity and diversity of small molecules. Polar and non-polar metabolites are typically separated using a biphasic solvent system such as methanol–chloroform–water. This approach allows for the partitioning of aqueous (hydrophilic) and organic (lipophilic) metabolites, which can be independently analyzed or recombined depending on the research objective. Rapid quenching of metabolic activity through snap freezing and cold extraction is critical to minimize post-sampling metabolic degradation or transformation.
- Standardization: Standardization is essential for ensuring reproducibility across NMR-based metabolomics studies, particularly when comparing data generated across different labs, instruments, or time points. Stringent protocols must govern every stage, including sample collection, storage conditions (e.g., temperature, duration, freeze–thaw cycles), solvent purity, and instrument calibration. Uniform data acquisition parameters-such as pulse sequence, relaxation delay, number of scans, and spectral width-must be maintained. Inclusion of internal standards like TSP or DSS for chemical shift referencing and concentration calibration further enhances the reliability and comparability of metabolomic data.
Data Processing and Statistical Analysis
Spectral Processing
Raw NMR data require meticulous processing to convert free induction decay (FID) signals into interpretable spectra. The first steps typically involve Fourier transformation, followed by manual or automated phase correction to adjust for phase errors introduced during acquisition. Baseline correction removes signal drift and artifacts, enhancing peak detection and quantification. Spectra are then referenced, usually to the chemical shift of a known internal standard such as TSP (0.00 ppm), and normalized to account for differences in sample concentration or volume. Depending on the analysis strategy, spectra may be binned into small chemical shift intervals (e.g., 0.01–0.04 ppm bins) to reduce data complexity, or peak deconvolution may be performed using software like Chenomx, NMRPipe, MNova, or Bruker's TopSpin to identify and quantify individual metabolites.
Multivariate Statistical Analysis
Metabolomics data are inherently high-dimensional and multicollinear, necessitating the use of multivariate statistical methods for meaningful interpretation.
- Unsupervised: Approaches such as Principal Component Analysis (PCA), reduce dimensionality while revealing intrinsic patterns, clusters, and outliers without prior group labeling. PCA provides an overview of data quality and sample distribution, often serving as a preliminary diagnostic tool.
- Supervised: Supervised techniques like Partial Least Squares Discriminant Analysis (PLS-DA) and Orthogonal PLS-DA (OPLS-DA) are used to model group differences and identify key metabolites contributing to class separation. These methods are especially valuable for biomarker discovery, as they enhance interpretability by highlighting variables that most strongly correlate with phenotypic differences.
- Validation: To ensure the validity and generalizability of these models, permutation testing and cross-validation (e.g., k-fold or leave-one-out) are employed to assess robustness and prevent overfitting.
Metabolite Identification and Pathway Analysis
Once spectral peaks have been assigned and quantified, the resulting list of metabolites is subjected to identification and biological contextualization. Compound identification is accomplished by comparing chemical shifts, coupling constants, and multiplicities with those in public databases such as the Human Metabolome Database (HMDB), the Kyoto Encyclopedia of Genes and Genomes (KEGG), and the Biological Magnetic Resonance Bank (BMRB). Confirmatory methods, such as spiking experiments with authentic standards, may be employed to validate ambiguous assignments. Identified metabolites are then mapped onto biochemical pathways to interpret their functional roles in physiological or pathological processes. Pathway analysis tools such as MetaboAnalyst, MetExplore, and Mummichog facilitate the integration of metabolite changes with known metabolic networks, enabling researchers to identify perturbed pathways, potential enzymatic targets, and broader systems-level effects. This step is critical for translating raw spectral data into biologically meaningful insights, which may ultimately lead to the discovery of novel biomarkers, therapeutic targets, or mechanistic hypotheses.
 Figure 3. Schematic workflow illustrating the steps of NMR based metabolomic studies coupled with chemometrics and pathway analysis. (1) Sample preparation and NMR tube filling (top left); (2) experimental parameters setting and data acquisition (top right); (3) data processing (middle left); (4) execution of multivariate statistical analysis (bottom right); (5) determination of metabolic pathways (bottom left). (Corsaro et al., 2022)
Figure 3. Schematic workflow illustrating the steps of NMR based metabolomic studies coupled with chemometrics and pathway analysis. (1) Sample preparation and NMR tube filling (top left); (2) experimental parameters setting and data acquisition (top right); (3) data processing (middle left); (4) execution of multivariate statistical analysis (bottom right); (5) determination of metabolic pathways (bottom left). (Corsaro et al., 2022)
Select Service
Applications in Biomarker Discovery
| Classification | Disease | Details |
|---|---|---|
| Clinical Diagnostics | Cancer | Distinct metabolic signatures in urine, plasma, and tissue for breast, prostate, and brain cancers. |
| Cardiovascular diseases | Lipoprotein profiling using NMR provides risk assessment and monitoring. | |
| Neurological disorders | Altered metabolic profiles in Alzheimer's, Parkinson's, and multiple sclerosis. | |
| Infectious diseases | Monitoring host and pathogen metabolic interactions. | |
| Drug Development | Pharmacometabolomics | Studies metabolic responses to drugs, aiding dose optimization and side effect prediction. |
| Toxicology | Detects early biomarkers of drug-induced toxicity. | |
| Mechanism of Action | Elucidates biochemical pathways influenced by therapeutic agents. | |
| Nutritional and Environmental Metabolomics | Dietary interventions | NMR tracks metabolic changes in response to diet and supplements. |
| Environmental exposures | Detects metabolic perturbations caused by pollutants, toxins, and stressors. | |
| Personalized Medicine | By profiling individual metabolic fingerprints, NMR contributes to personalized therapeutic strategies and preventive medicine. | |
Case Studies
Case 1: NMR metabolomics for non-invasive biomarker discovery in ESCC
This study used high-resolution 1H-NMR spectroscopy (600 MHz) to analyze serum, urine, and tissue samples from esophageal squamous cell carcinoma (ESCC) patients and healthy controls. The goal was to identify non-invasive metabolic biomarkers reflecting tumor-associated metabolic changes.
Creatine and glycine emerged as consistent markers across serum, urine, and cancerous tissues, linked to the disrupted glycine, serine, and threonine metabolism pathway. A combined serum-urine biomarker panel using these two metabolites achieved high diagnostic accuracy (AUC = 0.930), outperforming single-fluid models.
OPLS-DA pattern recognition of NMR spectra revealed clear group separations, validated by permutation tests, confirming the model's reliability. This work highlights the potential of NMR-based metabolomics for ESCC detection and paves the way for broader clinical applications in cancer diagnostics.
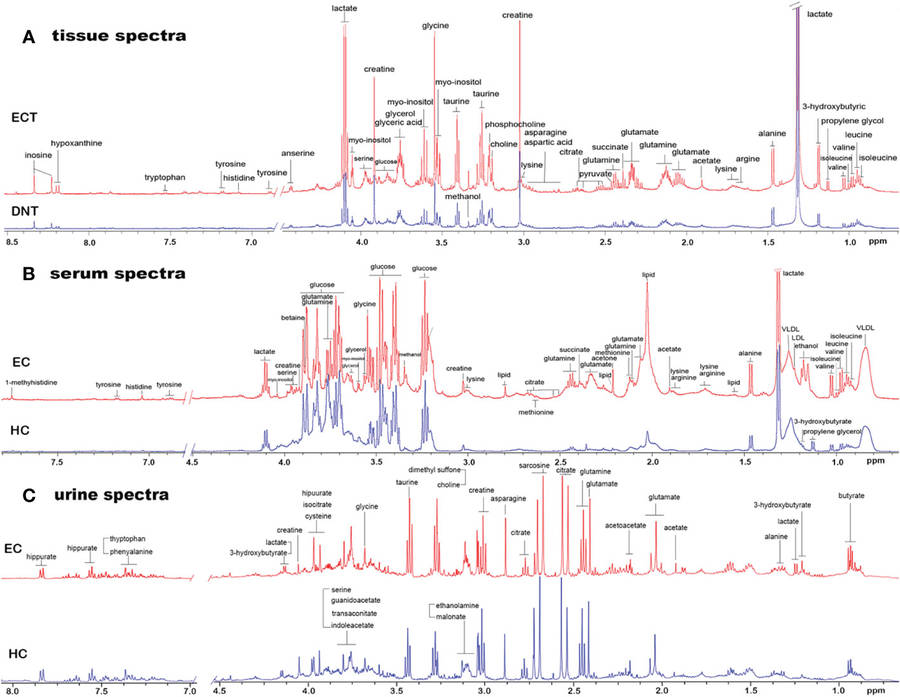 Figure 4. Representative 1D 1H NMR spectra of tissue, serum and urine from EC patients and controls. (A) Representative 600MHz NOESYPR1D 1H NMR spectra of esophageal tissue extract from ECT and DNT. (B) Representative 600MHz CMPG 1H NMR spectra of serum from EC and HC. (C) Representative 600MHz NOESYPR1D 1H NMR spectra of urine EC and HC. ECT, esophageal cancer tissue; DNT, distal noncancerous tissue; EC, esophageal cancer; HC, healthy control. (Ouyang et al., 2023)
Figure 4. Representative 1D 1H NMR spectra of tissue, serum and urine from EC patients and controls. (A) Representative 600MHz NOESYPR1D 1H NMR spectra of esophageal tissue extract from ECT and DNT. (B) Representative 600MHz CMPG 1H NMR spectra of serum from EC and HC. (C) Representative 600MHz NOESYPR1D 1H NMR spectra of urine EC and HC. ECT, esophageal cancer tissue; DNT, distal noncancerous tissue; EC, esophageal cancer; HC, healthy control. (Ouyang et al., 2023)
Case 2: Integrative GC-qMS and NMR metabolomics for breast cancer biomarker discovery
This study combined NMR and GC-qMS metabolomic analyses to distinguish breast cancer (BC) patients from healthy individuals using urine and tissue samples. Multivariate statistical models demonstrated strong diagnostic performance, with OPLS-DA models yielding high predictive accuracy (Q2 > 0.81 for both urine and tissue). Metabolic profiling revealed dysregulated pathways involving lactate, valine, aspartate, and glutamine, which are associated with cancer metabolism.
Five metabolites showed consistent alterations across both urine and tissue, suggesting potential non-invasive biomarkers. Overall, the dual-platform metabolomics approach offers valuable insights into BC biology and holds promise for improving diagnosis and monitoring in clinical oncology.
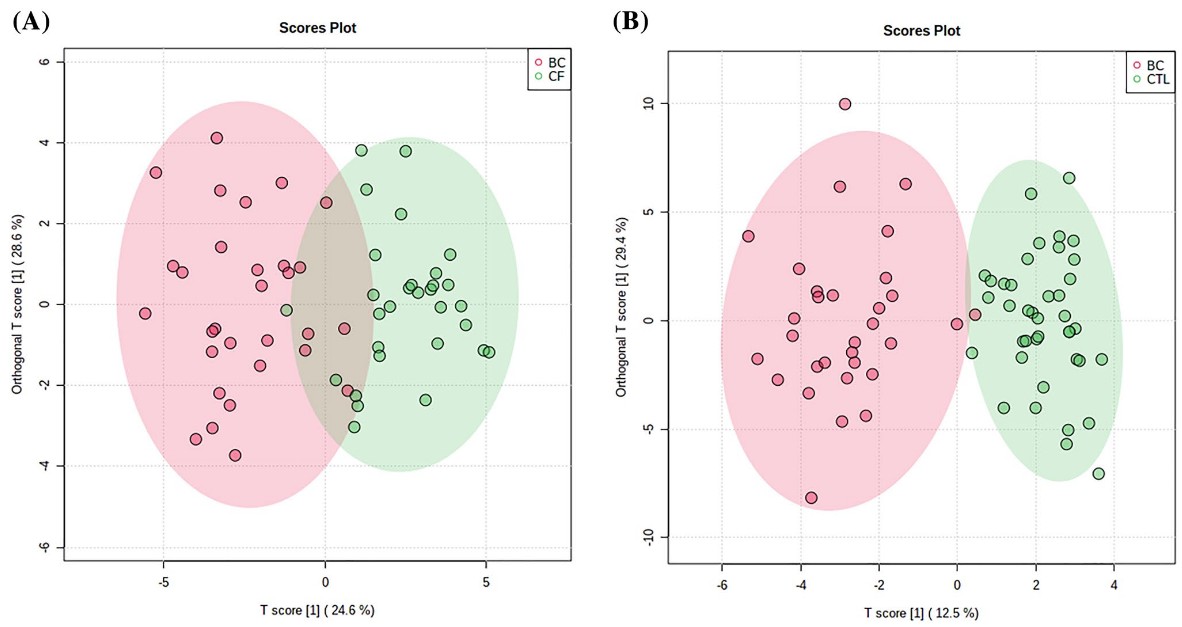 Figure 5. Orthogonal partial least-squares discriminant analysis (OPLS-DA) score plots between BC patients and cancer-free (CF) individuals for tissue (a) (R2Y = 0.888; Q2 = 0.813) and urine (b) (R2Y = 0.946 and Q2 = 0.910) samples obtained by NMR and GC-qMS analysis. (Silva et al., 2021)
Figure 5. Orthogonal partial least-squares discriminant analysis (OPLS-DA) score plots between BC patients and cancer-free (CF) individuals for tissue (a) (R2Y = 0.888; Q2 = 0.813) and urine (b) (R2Y = 0.946 and Q2 = 0.910) samples obtained by NMR and GC-qMS analysis. (Silva et al., 2021)
In summary, NMR spectroscopy has cemented its role as a non-destructive, quantitative, and structurally informative technique in metabolomics. Despite its lower sensitivity relative to MS, NMR offers unparalleled advantages in reproducibility, quantification, and structural elucidation. Its application in biomarker discovery spans clinical diagnostics, pharmacology, nutrition, and environmental health.
Partner with us at Creative Biostructure to unlock the full potential of NMR spectroscopy in your metabolomics research. Our cutting-edge NMR services deliver high-resolution, reproducible, and structurally insightful data to accelerate your biomarker discovery and translational science. Contact us today to elevate your research with precision and confidence.
References
- Corsaro C, Vasi S, Neri F, Mezzasalma AM, Neri G, Fazio E. NMR in metabolomics: from conventional statistics to machine learning and neural network approaches. Applied Sciences. 2022;12(6):2824.
- Fernandes WM, Harris N, Zamalloa A, et al. High-resolution magic angle spinning nuclear magnetic resonance spectroscopy of paired clinical liver tissue samples from hepatocellular cancer and surrounding region. IJMS. 2024;25(16):8924.
- Ouyang T, Ma C, Zhao Y, et al. 1H NMR-based metabolomics of paired tissue, serum and urine samples reveals an optimized panel of biofluids metabolic biomarkers for esophageal cancer. Front Oncol. 2023;13:1082841.
- Raja G, Jung Y, Jung SH, Kim TJ. 1H-NMR-based metabolomics for cancer targeting and metabolic engineering –A review. Process Biochemistry. 2020;99:112-122.
- Silva CL, Perestrelo R, Capelinha F, Tomás H, Câmara JS. An integrative approach based on GC-qMS and NMR metabolomics data as a comprehensive strategy to search potential breast cancer biomarkers. Metabolomics. 2021;17(8):72.