The plant kingdom has long served as a goldmine of bioactive compounds, providing an array of alkaloids, flavonoids, terpenes, polyphenols, and glycosides with therapeutic and industrial potential. Understanding the structure, function, and behavior of these complex natural products is essential for drug discovery, agriculture, nutraceutical development, and environmental research.
Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a critical tool in phytochemical research. Unlike other techniques that may require derivatization or destructive processing, NMR offers a non-destructive, quantitative, and highly informative approach for the detailed characterization of plant-based compounds. Its ability to analyze both pure compounds and complex mixtures—including crude extracts—makes it indispensable in modern natural products chemistry.
Creative Biostructure offers advanced NMR services, providing comprehensive solutions for the analysis of plant-based compounds and more.

Principle of NMR
At its core, NMR exploits the magnetic properties of certain atomic nuclei. Nuclei such as 1H, 13C, 15N, and 31P possess spin and generate a magnetic moment. When placed in a magnetic field, these nuclei can absorb specific radiofrequency (RF) radiation, transitioning between energy levels. The emitted signals upon relaxation are recorded as an NMR spectrum.
Key interpretive elements of NMR include:
- Chemical shift (δ): Reflects the electronic environment of the nucleus.
- Spin-spin coupling (J-coupling): Reveals connectivity between neighboring nuclei.
- Relaxation times (T1, T2): Offer insights into molecular dynamics.
- Multiplicity and integration: Indicate proton environments and quantities.
In the context of plant-based compounds—often structurally complex and stereochemically rich—these features allow for complete molecular structure elucidation, even without crystallization.
Unique Challenges
Plant matrices pose distinct challenges for structural chemists:
- Structural Complexity: Natural products often contain multiple chiral centers, conjugated systems, and functional group diversity.
- Low Concentration of Active Compounds: Active constituents may be present in trace amounts within complex matrices.
- Sample Purity: Crude plant extracts can contain hundreds of structurally similar molecules.
- Solubility Issues: Many plant-derived compounds have poor solubility in common solvents.
- Dynamic Behavior: Tautomerism, conformational flexibility, and exchange phenomena can complicate spectra.
NMR, especially when combined with modern hardware and pulse sequences, helps overcome these challenges by offering site-specific, multi-nuclear, and multidimensional analytical capabilities.
NMR in Characterizing Plant Natural Products
Isolation and Identification: Determining the Structure of Unknown Metabolites
During the isolation of plant-derived compounds, NMR serves as the gold standard for determining the structure of unknown metabolites. By analyzing the chemical shifts, coupling constants, and splitting patterns in NMR spectra, researchers can deduce the molecular framework, functional groups, and connectivity of atoms within a compound. For example, the characteristic chemical shifts of protons and carbons in NMR spectra can reveal the presence of aromatic rings, aliphatic chains, and various substituents. Techniques such as 2D NMR (e.g., COSY, HSQC, HMBC) further enhance the ability to elucidate complex structures by providing correlations between different nuclei, thereby establishing the complete molecular architecture.
Dereplication: Rapid Identification of Known Compounds to Avoid Re-Isolation
Dereplication is an essential step in natural product research aimed at identifying known compounds quickly and efficiently to avoid redundant isolation efforts. NMR spectra, particularly 1H and 13C NMR, provide unique and reproducible fingerprints for each compound. By comparing the NMR data of isolated compounds with reference spectra available in databases or literature, researchers can rapidly identify known metabolites. This approach saves time and resources, allowing researchers to focus on novel compounds. Modern NMR instruments equipped with automated dereplication software can even match spectra in real-time, streamlining the identification process.
Structural Revision: Re-examining Previously Misassigned Structures with Improved Techniques
Advancements in NMR techniques and instrumentation have led to more accurate and detailed structural analyses. This progress has prompted the re-examination of previously assigned structures, especially those that may have been misinterpreted due to limitations in earlier analytical methods. With improved resolution, sensitivity, and multidimensional NMR experiments, researchers can now identify errors in past assignments and correct them. For example, ambiguities in the stereochemistry or connectivity of certain functional groups that were once challenging to resolve can now be clarified with modern NMR approaches, ensuring the accuracy of structural information in the literature.
Stereochemical Determination: Assigning Relative and Absolute Configurations
Stereochemistry is a critical aspect of natural product structure elucidation, as it significantly influences the biological activity and properties of a compound. NMR provides powerful tools for determining both relative and absolute configurations. The relative configuration can often be inferred from coupling constants and chemical shifts, while absolute configuration can be established using advanced techniques such as Mosher's method or chiral shift reagents. These methods involve comparing the NMR spectra of the natural product with those of known chiral standards, allowing researchers to accurately assign the spatial arrangement of atoms in chiral centers.
Select Service
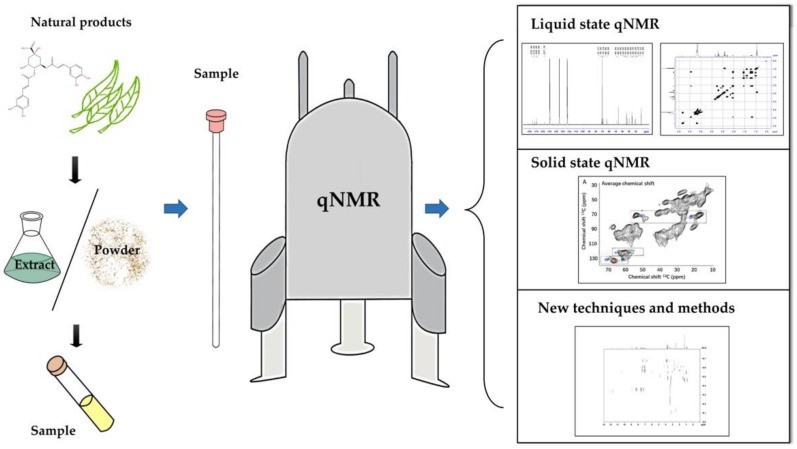
Quantitative NMR (qNMR) in Phytochemistry
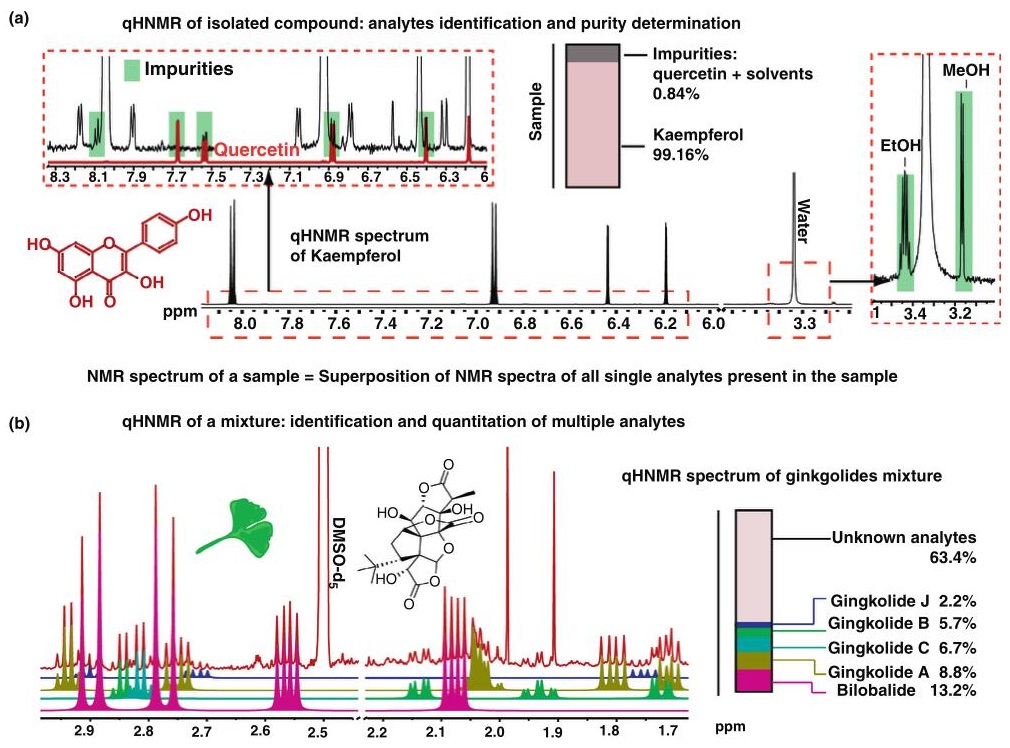
Quantitative NMR (qNMR) is a powerful analytical technique used in phytochemistry for the precise quantification of compounds in plant extracts. It leverages the inherent quantitative nature of NMR, where the signal intensity is directly proportional to the concentration of the analyte. This method is particularly useful for determining the purity of reference materials, quantifying active ingredients, and assessing the composition of complex mixtures. qNMR can achieve high accuracy and precision, often below 5%, with a linear response over a wide concentration range (10 μM to 1 M) and detection limits as low as 1 μM. It is commonly used in the quality control of botanical dietary supplements and herbal medicines, providing an unbiased evaluation of sample composition and simultaneous quantification of multiple compounds.
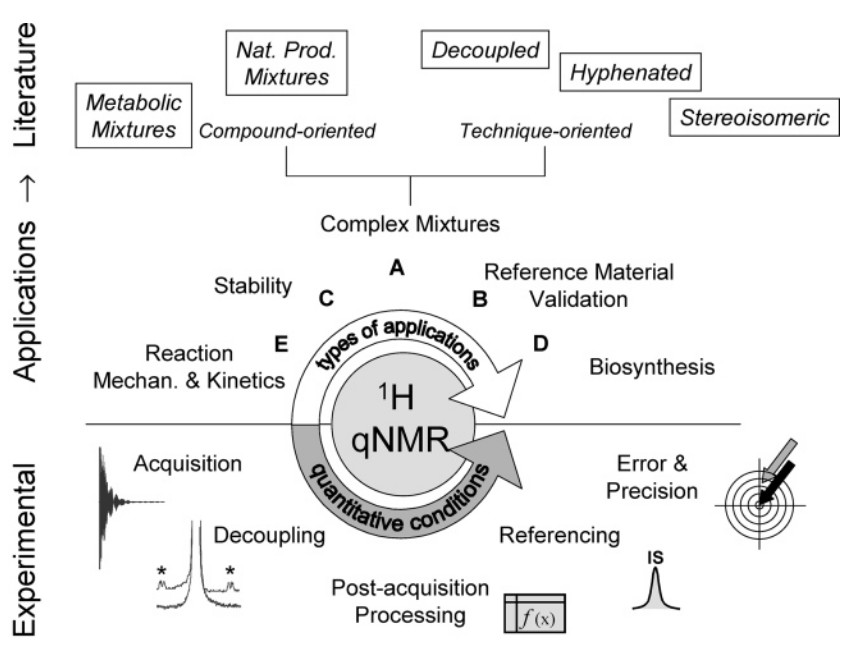 Figure 1. Overview of quantitative 1H NMR in development and potential of a method for natural products analysis. Based on a general overview of experimental parameters (bottom) that lead to "quantitative conditions", reported applications of the main areas of 1H NMR analysis (middle) is compiled with particular focus on analytical procedures for natural products (top). (Pauli et al., 2005)
Figure 1. Overview of quantitative 1H NMR in development and potential of a method for natural products analysis. Based on a general overview of experimental parameters (bottom) that lead to "quantitative conditions", reported applications of the main areas of 1H NMR analysis (middle) is compiled with particular focus on analytical procedures for natural products (top). (Pauli et al., 2005)
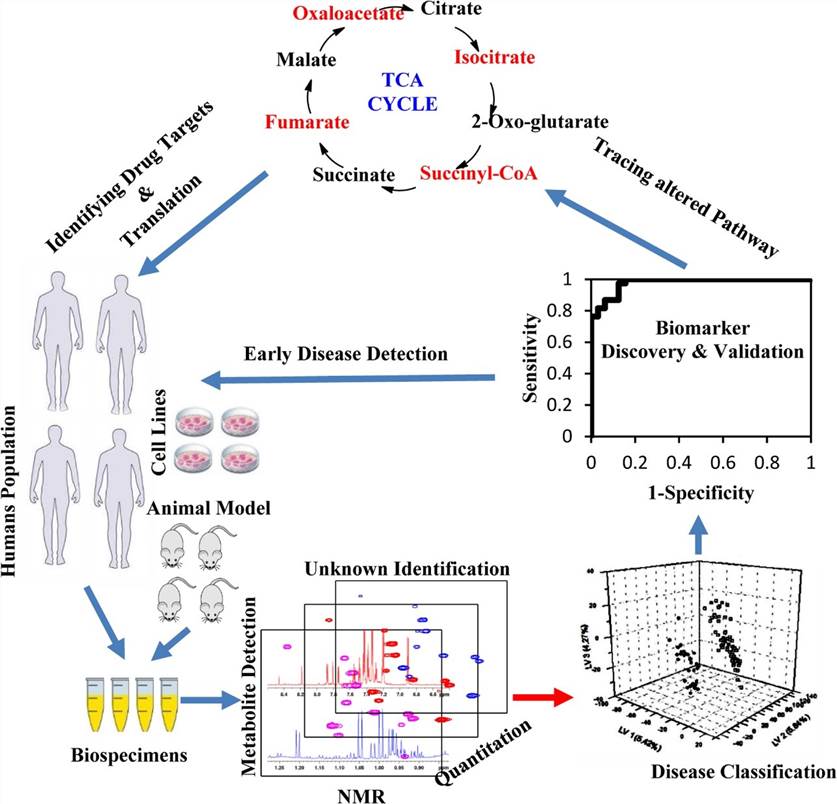
Metabolomics and Chemotaxonomy via NMR
NMR-based metabolomics is a rapidly growing field that utilizes NMR spectroscopy to study the metabolite profiles of biological samples. This technique is highly reproducible and provides a comprehensive, unbiased representation of the metabolome, making it ideal for studying plant metabolites. NMR can detect and quantify a wide range of metabolites in plant extracts, providing insights into primary and secondary metabolism. For example, qNMR has been used to monitor the changing content of sugars and organic acids in fruits over time, helping to improve fruit quality. In chemotaxonomy, NMR is used to classify plants based on their chemical profiles, which can reveal evolutionary relationships and identify unique metabolic pathways in different plant species.
Bioavailability and Interaction Studies
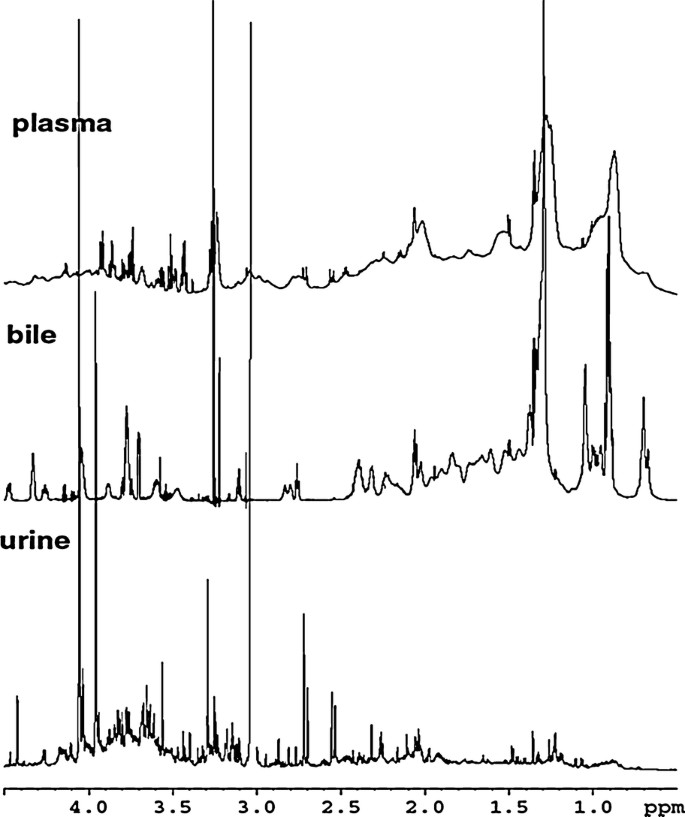
NMR is also a valuable tool for studying the bioavailability and interactions of plant-derived compounds. Bioavailability studies using NMR can track the absorption, distribution, metabolism, and excretion (ADME) of active ingredients in biological systems. For example, NMR has been used to analyze the metabolite profiles of biofluids such as urine, blood plasma, and bile, providing insights into the metabolic fate of plant-derived compounds in the body. Additionally, NMR can be used to study interactions between plant metabolites and other biomolecules, such as proteins and lipids. This information is crucial for understanding the biological activity and therapeutic potential of plant-derived compounds.
Essential NMR Methods for Plant Compounds
| Methods | Description | |
|---|---|---|
| 1D NMR Spectroscopy | 1H NMR | First-line technique for identifying proton environments, multiplicities, and integrations. |
| 13C NMR | Reveals carbon skeleton; DEPT editing distinguishes CH, CH2, CH3. | |
| Selective 1D TOCSY or NOE | Useful in complex overlapping regions. | |
| 2D NMR Spectroscopy | COSY (Correlation Spectroscopy) | Shows 1H–1H spin systems. |
| HSQC/HMQC (Heteronuclear Single Quantum Coherence) | Correlates 1H and directly bonded 13C atoms. | |
| HMBC (Heteronuclear Multiple Bond Correlation) | Identifies long-range C–H correlations (2–3 bonds), crucial for skeleton assembly. | |
| NOESY/ROESY | Offers spatial proximity data; essential for stereochemical assignments. | |
| TOCSY | Maps whole spin systems; effective in identifying sugar moieties and peptide sequences. | |
| Advanced and Specialized Techniques | qNMR (Quantitative NMR) | Enables absolute quantitation without external calibration curves. |
| Diffusion-Ordered Spectroscopy (DOSY) | Separates components by size/diffusion behavior—useful in mixture analysis. | |
| Solid-State NMR | Applied to insoluble plant materials like lignin or cellulose. | |
| Chiral Derivatization Agents (e.g., Mosher's reagents) | Determine absolute configuration. | |
Compound Classes Ideal for NMR Analysis
Flavonoids: Distinguishing Glycosylation Patterns and Substitution Positions
Flavonoids are a diverse class of plant-derived natural products with a wide range of biological activities. NMR is particularly well-suited for studying these compounds due to their characteristic aromatic structures and functional groups. The chemical shifts of protons and carbons in the aromatic rings can provide information about substitution patterns, while the coupling constants can reveal the positions of hydroxyl and methoxy groups. Additionally, NMR can distinguish between different glycosylation patterns, such as the attachment of sugar moieties to different positions on the flavonoid core, which is crucial for understanding their bioavailability and activity.
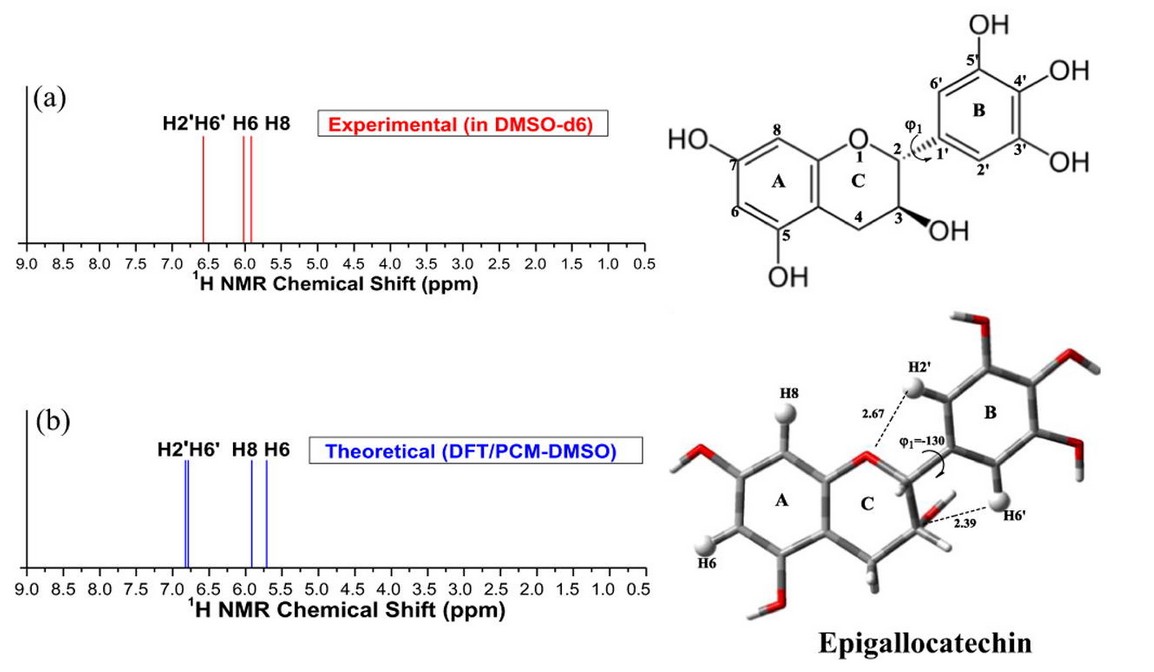 Figure 2. Structural analysis of flavonoids in solution through DFT 1H NMR chemical shift calculations—Epigallocatechin. (De Souza et al., 2017)
Figure 2. Structural analysis of flavonoids in solution through DFT 1H NMR chemical shift calculations—Epigallocatechin. (De Souza et al., 2017)
Alkaloids: Elucidating Fused Ring Systems and Stereochemistry
Alkaloids are complex nitrogen-containing natural products with intricate ring systems and often multiple chiral centers. NMR is invaluable for elucidating the structures of these compounds, particularly in resolving fused ring systems and determining stereochemistry. The characteristic chemical shifts of nitrogen-bearing protons and carbons, along with long-range coupling constants, help in mapping out the ring connectivity. Furthermore, NMR techniques such as NOE (Nuclear Overhauser Effect) spectroscopy can provide spatial information about the relative positions of atoms, aiding in the determination of the three-dimensional structure and stereochemistry.
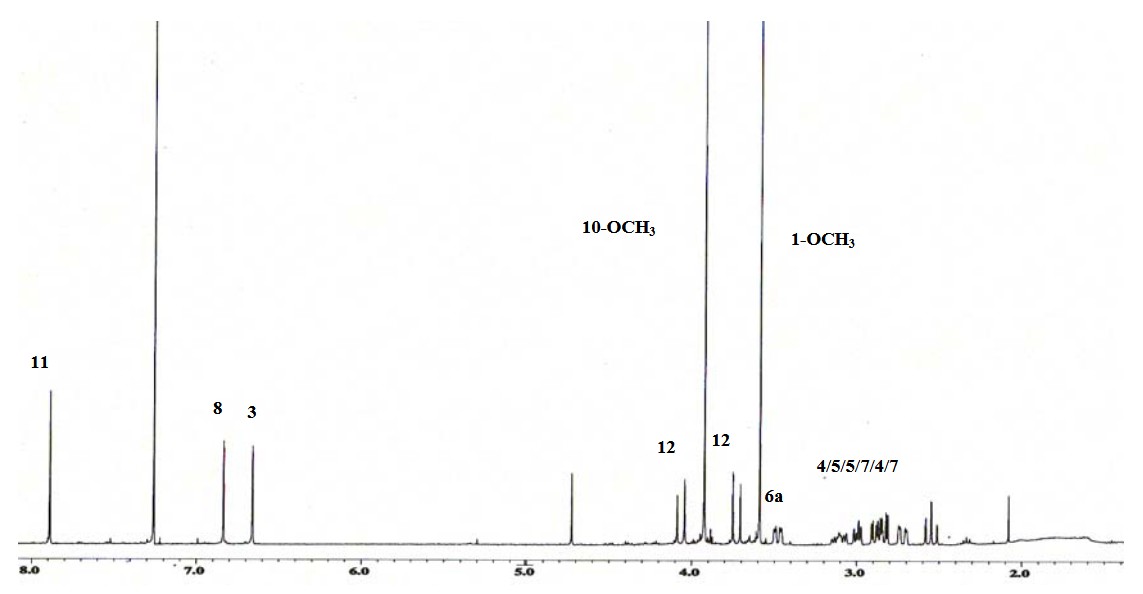 Figure 3. 1H NMR spectrum of an alkaloid isolated from Alseodaphne perakensis. (Nafiah et al., 2011)
Figure 3. 1H NMR spectrum of an alkaloid isolated from Alseodaphne perakensis. (Nafiah et al., 2011)
Terpenoids: Resolving Isomerism in Complex Frameworks
Terpenoids are a large and structurally diverse class of natural products with complex carbon skeletons. NMR is essential for resolving isomerism in these compounds, as it can distinguish between different isomers based on their unique NMR spectra. The chemical shifts of protons and carbons in the terpenoid framework, along with coupling constants, provide information about the arrangement of double bonds, methyl groups, and other functional groups. Advanced techniques such as ROESY (Rotating Frame Overhauser Effect Spectroscopy) can further elucidate the spatial relationships between atoms, helping to differentiate between stereoisomers and positional isomers.
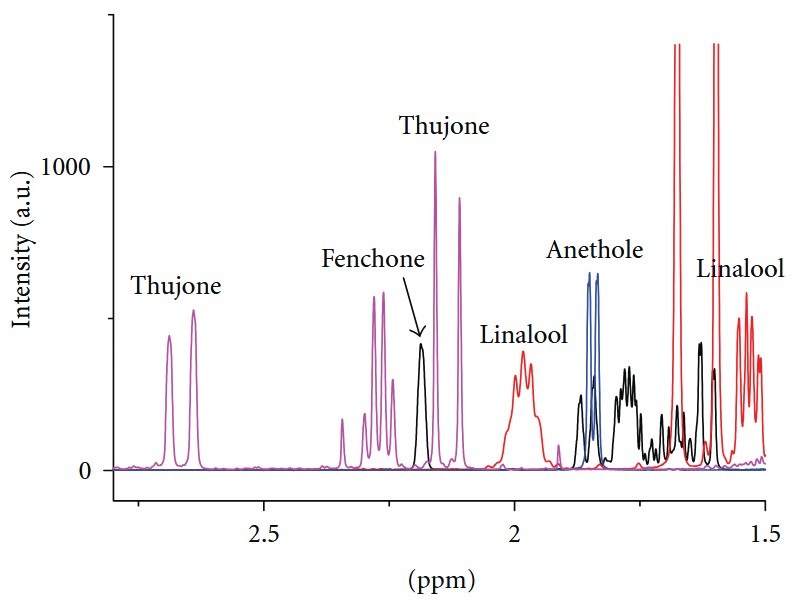 Figure 4. NMR spectra (aliphatic range) of common terpenes occurring in absinthe. (Monakhova et al., 2011)
Figure 4. NMR spectra (aliphatic range) of common terpenes occurring in absinthe. (Monakhova et al., 2011)
Phenolics: Identifying Conjugation and Oxidation States
Phenolic compounds are widely present in plants and exhibit various biological activities. NMR is highly effective in characterizing these compounds by identifying conjugation patterns and oxidation states. The chemical shifts of protons and carbons in aromatic rings can reveal the presence and extent of conjugation, while the signals of hydroxyl and carbonyl groups provide information about the oxidation state. For example, the downfield shift of aromatic protons in conjugated systems and the characteristic chemical shifts of quinone or catechol groups can be easily detected and interpreted using NMR.
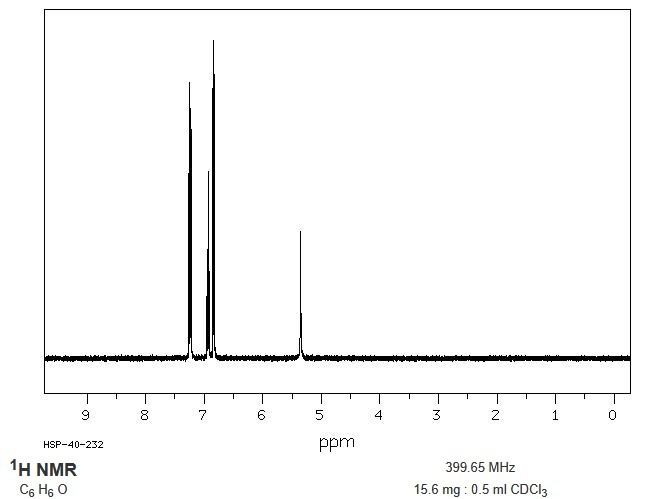 Figure 5. 1H NMR spectrum of phenol, 400 MHz in CDCl3. (chemicalbook.com)
Figure 5. 1H NMR spectrum of phenol, 400 MHz in CDCl3. (chemicalbook.com)
Select Service
Related Reading
Case Studies
Case 1: 13C CPMAS NMR for curcumin supplement quality control
Turmeric, a traditional Indian spice, has gained global fame thanks to curcumin, its powerful anti-inflammatory compound. However, curcumin supplements face two big issues: poor water solubility and counterfeiting with synthetic curcumin.
This study proposes using 13C CPMAS NMR (a solid-state NMR method) combined with GIPAW computations to verify supplement quality. It helped identify a polymorphic form affecting curcumin's solubility, and a faked supplement containing synthetic curcumin. The approach is particularly advantageous because it enables direct analysis of capsule or tablet contents without requiring complex sample preparation. Its effectiveness was further validated by PXRD and HPLC, confirming its potential for routine quality assurance in supplement manufacturing.
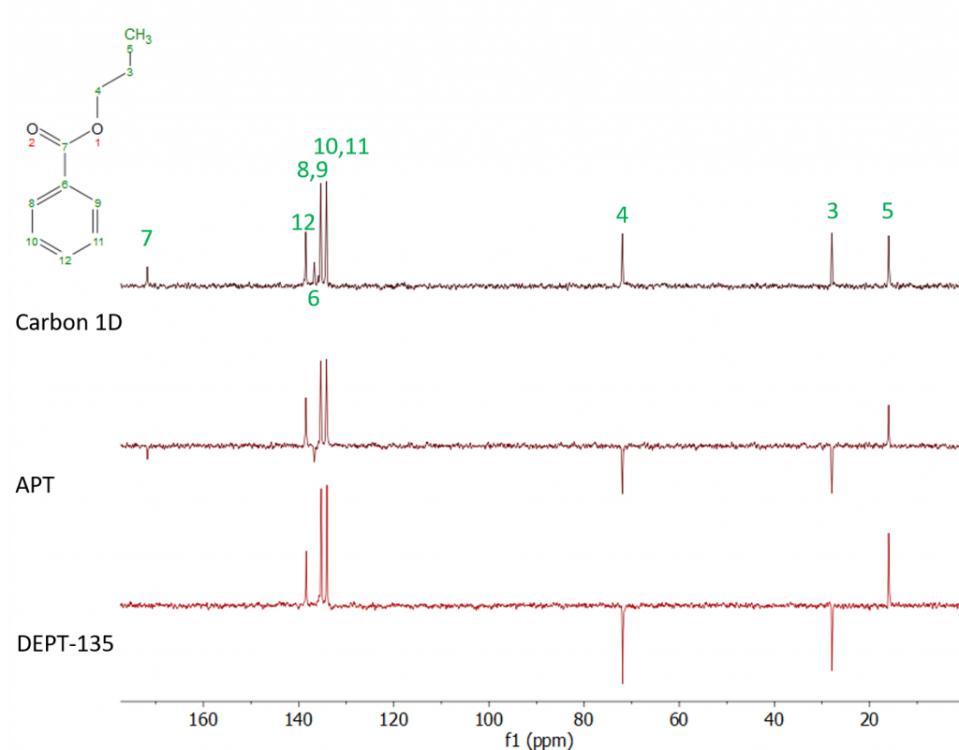
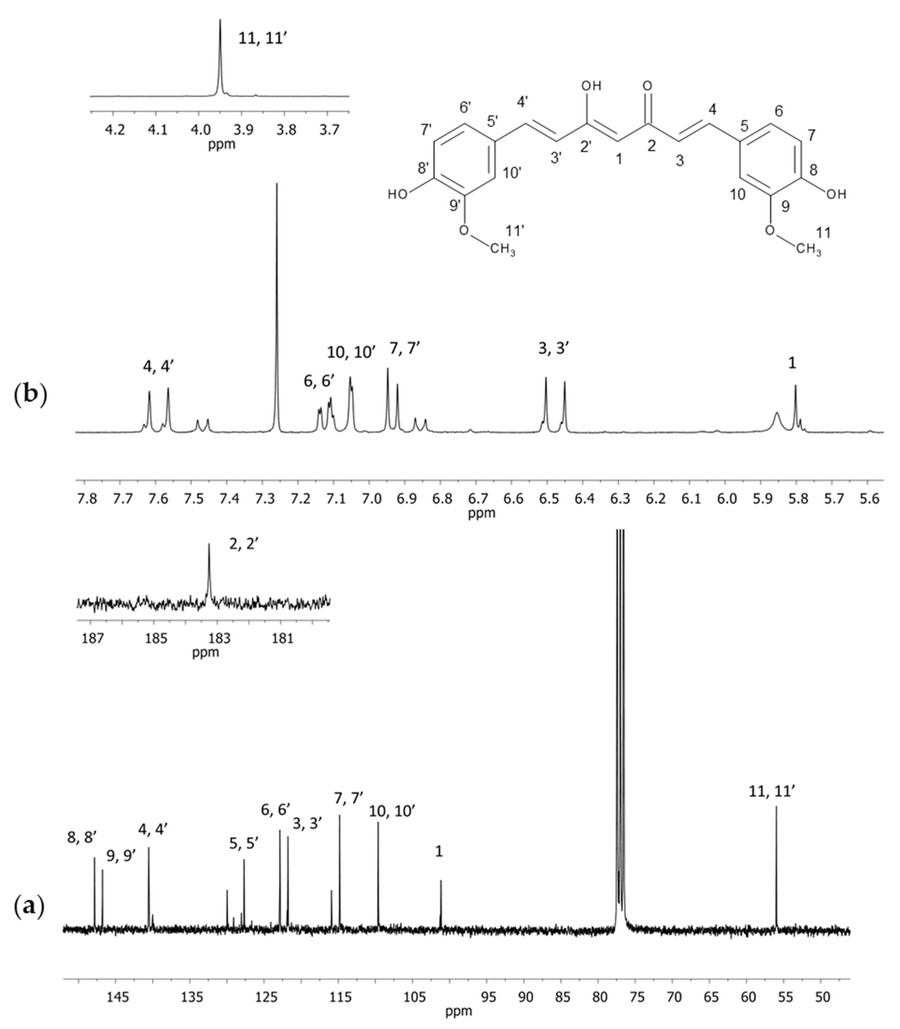 Figure 6. NMR spectra of curcumin in CDCl3 (a) 13C and (b) 1H with an extended section of 4.7–4.2 ppm (a) and 181–187 ppm (b). Numbers correspond with carbon or hydrogen atoms in curcumin structure. (Siudem et al., 2023)
Figure 6. NMR spectra of curcumin in CDCl3 (a) 13C and (b) 1H with an extended section of 4.7–4.2 ppm (a) and 181–187 ppm (b). Numbers correspond with carbon or hydrogen atoms in curcumin structure. (Siudem et al., 2023)
Case 2: NMR and in silico isolation of resveratrol & ε-viniferin via NADES
This study demonstrates the application of solid-state NMR spectroscopy to support the characterization and analysis of stilbenoid compounds extracted from grapevine canes, which are a rich source of bioactive molecules. In conjunction with NMR, an in-silico method based on the Conductor-like Screening Model for Real Solvents (COSMO-RS) was utilized to optimize the extraction process, particularly for resveratrol and ε-viniferin, using natural deep eutectic solvents (NADES) as environmentally sustainable alternatives to conventional solvents.
By integrating COSMO-RS with high-performance countercurrent chromatography (HPCCC) and employing ultrasonic-assisted extraction, the study achieved enhanced extraction efficiency. Solid-state NMR played a crucial role in confirming the structural integrity and composition of the isolated compounds, reinforcing the effectiveness of this computational and experimental workflow.
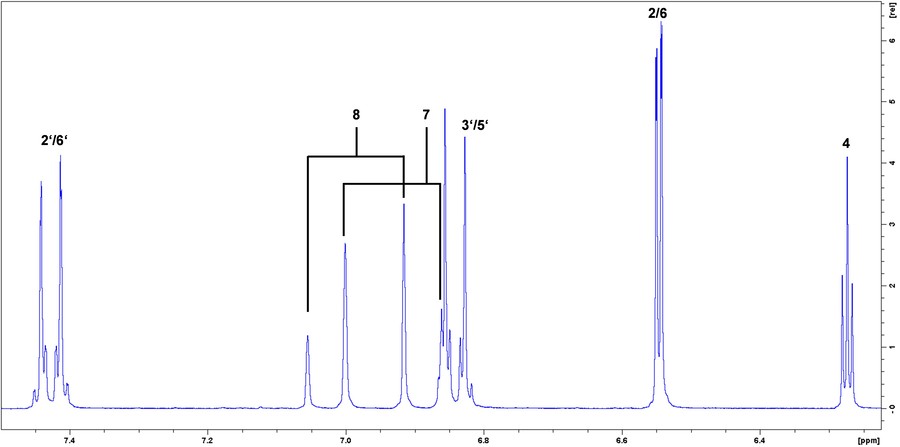 Figure 7. 1H-NMR spectrum of trans-resveratrol. (Kiene et al., 2023)
Figure 7. 1H-NMR spectrum of trans-resveratrol. (Kiene et al., 2023)
In summary, NMR spectroscopy is an unparalleled tool in the arsenal of natural products and plant compound researchers. From primary structure elucidation to quantification, metabolomics, and interaction studies, it offers unmatched specificity and depth of information.
At Creative Biostructure, we offer a full suite of NMR services tailored for plant-based studies, including advanced 1D/2D NMR techniques, solid-state NMR, and quantitative NMR. Whether you're decoding the structure of a novel compound, validating a supplement, or conducting metabolomics, our expert team is here to support every stage of your project.
References
- De Souza LA, Tavares WMG, Lopes APM, Soeiro MM, De Almeida WB. Structural analysis of flavonoids in solution through DFT 1H NMR chemical shift calculations: Epigallocatechin, Kaempferol and Quercetin. Chemical Physics Letters. 2017;676:46-52.
- Kiene M, Zaremba M, Fellensiek H, et al. In silico-assisted isolation of trans-resveratrol and trans-ε-viniferin from grapevine canes and their sustainable extraction using natural deep eutectic solvents (Nades). Foods. 2023;12(22):4184.
- Monakhova YB, Kuballa T, Lachenmeier DW. Rapid determination of total thujone in absinthe using 1H NMR spectroscopy. Jackowski K, ed. International Journal of Spectroscopy. 2011;2011(1):171684.
- Nafiah MA, Mukhtar MR, Omar H, et al. N-cyanomethylnorboldine: a new aporphine isolated from alseodaphne perakensis (Lauraceae). Molecules. 2011;16(4):3402-3409.
- Pauli GF, Jaki BU, Lankin DC. Quantitative 1H NMR: development and potential of a method for natural products analysis. J Nat Prod. 2005;68(1):133-149.
- Siudem P, Szeleszczuk Ł, Zielińska A, Paradowska K. 13C CPMAS NMR as an alternative method to verify the quality of dietary supplements containing curcumin. Molecules. 2023;28(8):3442.