Key Takeaways: Quick Overview of Epitope Tags
What is an epitope tag: A short, genetically engineered peptide sequence used to aid detection and purification of proteins.
Most used tags: V5, FLAG, HA, Myc.
Detection method: Typically detected using epitope tag-specific antibodies.
Key applications: Protein purification, subcellular localization, and protein-protein interaction analysis.
What Are Epitope Tags?
Epitope tags are short peptide sequences genetically attached to recombinant proteins to facilitate detection and purification. They are fused to the N- or C-terminus of a protein and serve as a universal recognition site for commercial antibodies. Unlike functional or affinity tags, epitope tags do not necessarily contribute to protein activity or binding, making them ideal for visualization and isolation.
Epitope tags eliminate the need for target-specific antibodies, facilitating the study of novel or poorly immunogenic proteins. They simplify the purification process and improve protein tracking in cells, facilitating applications in structural biology, drug discovery and proteomics.
 Figure 1. Step-by-step illustration of how epitope tags are inserted, expressed in fusion proteins, and used for detection or purification. (Creative Biostructure)
Figure 1. Step-by-step illustration of how epitope tags are inserted, expressed in fusion proteins, and used for detection or purification. (Creative Biostructure)
Epitope Tag vs Affinity Tag
| Feature | Epitope Tag | Affinity Tag |
|---|---|---|
| Recognition | Antibody-specific | Ligand-specific (e.g., Ni, glutathione) |
| Tag Size | Small peptides | Can be small or large proteins |
| Main Function | Detection, immunoprecipitation | Purification |
| Use Cases | Western blot, IP, localization | Column purification |
Use epitope tags when high sensitivity in detection is required, and affinity tags when purification is the main goal. For a comprehensive overview of available protein tags and their research applications, you can explore this detailed guide on protein tags.
Types of Epitope Tags
Epitope tags are highly customizable and fall into several categories based on their size, structure and functional properties. These tags are typically classified as peptide tags, protein/fluorescent tags, and compared based on key performance characteristics such as detection method and potential interference with protein function.
Peptide Tags
FLAG Epitope Tag
Overview: The FLAG tag is a hydrophilic, eight-amino-acid peptide that offers exceptionally low structural interference, making it ideal for sensitive applications like protein crystallography or in vivo expression. Its design allows for calcium-dependent binding when used with certain anti-FLAG antibodies (e.g., M1 clone), providing an additional purification control mechanism. FLAG is especially popular in mammalian and insect systems, and its corresponding antibodies (e.g., M2 clone) are widely validated for Western blotting, IP, IHC, and ELISA. The FLAG tag is also commonly incorporated into tandem affinity purification (TAP) systems to achieve high-purity protein complexes.
Sequence: DYKDDDDK
Applications: Calcium-dependent binding, low interference with protein structure
HA Tag (Hemagglutinin)
Overview: The HA epitope tag originates from the human influenza hemagglutinin protein. It is known for its high immunogenicity, making it easily recognized by robust monoclonal antibodies. Because of this, HA is frequently used in protein localization, cell-based expression, and pull-down assays. The tag performs well in Western blotting, IP, ChIP, and IF applications. It is often used in dual-tagging strategies where one tag is used for purification and the HA tag is used for detection. Due to its proven performance in multiple model organisms, HA remains a popular choice in functional genomics studies.
Sequence: YPYDVPDYA
Applications: Immunoprecipitation, co-IP, immunofluorescence
Myc Epitope Tag
Overview: The Myc tag, derived from the human c-Myc oncogene, is one of the most frequently used epitope tags in molecular biology. It is particularly advantageous for proteins lacking commercial antibodies, allowing researchers to leverage anti-Myc reagents across various detection formats. Myc tags are commonly placed at the N- or C-terminal ends of recombinant proteins and work reliably in Western blot, immunoprecipitation, cell imaging, and ELISA. Their relatively neutral sequence ensures minimal impact on protein activity or folding, making them suitable for structural and functional studies alike.
Sequence: EQKLISEEDL
Applications: Ideal for detection in the absence of specific antibodies
V5 Epitope Tag
Overview: The V5 epitope tag is derived from a small epitope of the P and V proteins of simian virus 5 (SV5). Its relatively short and neutral amino acid sequence minimizes interference with protein folding and function. V5 tags are widely used in affinity purification workflows, particularly in mammalian expression systems, due to their high specificity and low immunogenicity. The tag is readily detected by anti-V5 monoclonal antibodies and is compatible with applications such as Western blotting, co-immunoprecipitation, and fluorescence-based assays. It is often paired with other tags like His for dual-tag strategies.
Sequence: GKPIPNPLLGLDST
Applications: Affinity purification, Western blot, immunoprecipitation (IP)
Protein & Fluorescent Tags
GST (Glutathione-S-Transferase)
Overview: GST is a 26 kDa protein tag that significantly increases the solubility of target recombinant proteins, especially when expressed in bacterial systems like E. coli. Beyond improving solubility, GST enables easy affinity purification via its strong binding to glutathione-coated resins. It also functions as a fusion partner that may improve protein folding or stability. However, due to its size, GST may interfere with downstream applications or require removal via protease cleavage after purification. Despite this, its robustness and simplicity make it a go-to tag in protein expression and purification workflows.
Function: Enhances solubility and facilitates purification through glutathione resin.
GFP / mCherry
Overview: Green fluorescent protein (GFP) and its red-shifted variant mCherry are fluorescent protein tags that allow real-time visualization of protein dynamics in living cells. Unlike peptide tags, these proteins emit intrinsic fluorescence, eliminating the need for secondary detection reagents. GFP is widely used for studying subcellular localization, gene expression, and protein mobility, while mCherry offers improved photostability and is suitable for multiplexed imaging. These tags are indispensable in cell biology, developmental biology, and synthetic biology, especially when combined with live-cell microscopy and fluorescence resonance energy transfer (FRET) assay applications.
Function: Used for real-time protein tracking and localization in live-cell imaging.
Comparison of Commonly Used Epitope Tags
| Tag | Sequence | Origin/Features | Advantages | Limitations |
|---|---|---|---|---|
| FLAG | DYKDDDDK | Synthetic peptide; includes enterokinase cleavage site | Small size (8 amino acids), high specificity, reversible elution; minimal impact on protein function due to hydrophilicity | Potential interference with protein function when fused at N- or C-terminus; possible post-translational modifications affecting antibody recognition |
| HA | YPYDVPDYA | Derived from human influenza hemagglutinin (amino acids 98–106) | Small size (9 amino acids), high specificity, suitable for immunofluorescence and immunoprecipitation | May be cleaved during apoptosis, leading to loss of immunoreactivity; potential cross-reactivity in certain cell lines |
| Myc | EQKLISEEDL | Derived from human c-Myc protein (amino acids 410–419) | Small size (10 amino acids), widely used for detection when specific antibodies are unavailable | Antibody cross-reactivity with endogenous Myc proteins; not recommended for fusion directly after signal peptides |
| V5 | GKPIPNPLLGLDST | Derived from simian virus 5 (SV5) P and V proteins | Suitable for detection in various expression systems; often used in combination with His-tag for purification | Larger size (14 amino acids) may affect protein folding; potential cross-reactivity in mammalian systems |
| GFP | 238 amino acids (~27 kDa) | Derived from Aequorea victoria jellyfish; emits green fluorescence upon excitation | Enables real-time visualization of protein localization and dynamics in live cells; does not require additional substrates or cofactors | Large size may interfere with protein folding or function; potential for oligomerization; fluorescence can be sensitive to environmental conditions |
Applications of Epitope Tags
1. Monitoring Protein Expression
Epitope tags are widely used to monitor the expression levels of recombinant proteins. Techniques such as Western blotting and ELISA employ antibodies specific to tags like FLAG, HA, or Myc to detect tagged proteins. For instance, in HEK293 cells expressing FLAG-tagged viral proteins, anti-FLAG antibodies can confirm expression efficiency through Western blot analysis. The small size of these tags minimizes interference with protein folding and function, ensuring accurate detection.
2. Cellular and Subcellular Localization
Determining the localization of proteins within cells is crucial for understanding their function. Immunofluorescence (IF) and immunohistochemistry (IHC) techniques utilize epitope tags to visualize protein distribution. For example, inserting an HA tag into different regions of the Na,K-ATPase α-subunit allows researchers to analyze its topology by identifying intracellular or extracellular domains using specific antibodies. However, the insertion site of the tag can affect protein function, necessitating functional assays to validate results.
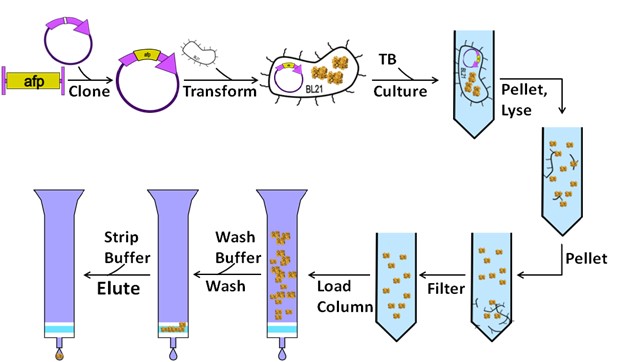
3. Protein Purification
Epitope tags facilitate the purification of recombinant proteins through affinity chromatography. The FLAG tag, for instance, binds to anti-FLAG M2 antibodies and can be eluted using EDTA or FLAG peptides, achieving purities up to 90%. Combining HA and His tags enables tandem purification strategies, reducing non-specific binding and enhancing purity. Nonetheless, considerations such as antibody cost and potential protein denaturation during elution must be addressed.
4. Protein-Protein Interaction Studies
Understanding protein interactions is vital for elucidating cellular pathways. Co-immunoprecipitation (Co-IP) techniques employ epitope tags like Myc or FLAG to capture protein complexes, which are then analyzed by mass spectrometry. For example, using Myc-tagged proteins in Co-IP experiments can identify interacting partners, providing insights into signaling networks. Tandem affinity purification (TAP), combining tags such as FLAG and HA, offers a two-step purification process that significantly reduces background noise, facilitating the study of complex protein assemblies.
5. Functional Genomics and Transgenic Research
Epitope tagging is instrumental in functional genomics and transgenic studies. CRISPR/Cas9 technology allows for the insertion of tags like FLAG into endogenous genes, enabling the study of proteins under physiological conditions. In gene therapy research, incorporating HA tags into AAV vectors permits tracking the expression and distribution of therapeutic proteins in vivo. These applications underscore the versatility of epitope tags in advancing our understanding of gene function and therapeutic interventions.
Case Study: FLAG Tag for Vaccine-Grade Protein Purification in Plants
In a plant-based recombinant protein expression system using Nicotiana benthamiana, researchers constructed several expression vectors to produce a multi-epitope peptide (MEP) derived from the Japanese encephalitis virus. Among these, the version tagged with a FLAG sequence at the N-terminus showed the highest expression level and superior purification efficiency.
Western blot analysis revealed that the FLAG-tagged construct produced a signal approximately 10 times stronger than alternative tags. Moreover, FLAG-based purification resulted in higher recovery of target protein with reduced contamination from host proteins.
The purified FLAG-tagged MEP protein was tested in mice and successfully induced a strong IgG antibody response, with antibody levels 10-20 times higher than controls. It also showed measurable virus-neutralizing activity, confirming the functional integrity of the purified protein.
This case illustrates that the FLAG tag can significantly enhance both the yield and purity of target proteins, while also preserving their biological activity, making it a valuable choice for vaccine development and therapeutic research.
 Figure 2. Comparison of JEV-MEP expression before and after FLAG-tag purification using SDS-PAGE and immunoblotting. (Jung J W, et al., 2023)
Figure 2. Comparison of JEV-MEP expression before and after FLAG-tag purification using SDS-PAGE and immunoblotting. (Jung J W, et al., 2023)
Case Study: FLAG Tag Enables Endogenous Protein Detection in Knock-In Mice
In a recent study, researchers employed the i-GONAD genome editing method to insert a FLAG (DYKDDDDK) tag at the N-terminus of the endogenous CaMKIIα and CaMKIIβ genes in mice. Using AlphaFold2 for protein structure prediction, they carefully selected a tagging site that preserved the natural function of the proteins. The resulting knock-in mice exhibited normal development and fertility. FLAG-tagged proteins were effectively detected with commercial antibodies through Western blot, immunoprecipitation, and immunohistochemistry, allowing reliable analysis of endogenous protein expression and interactions.
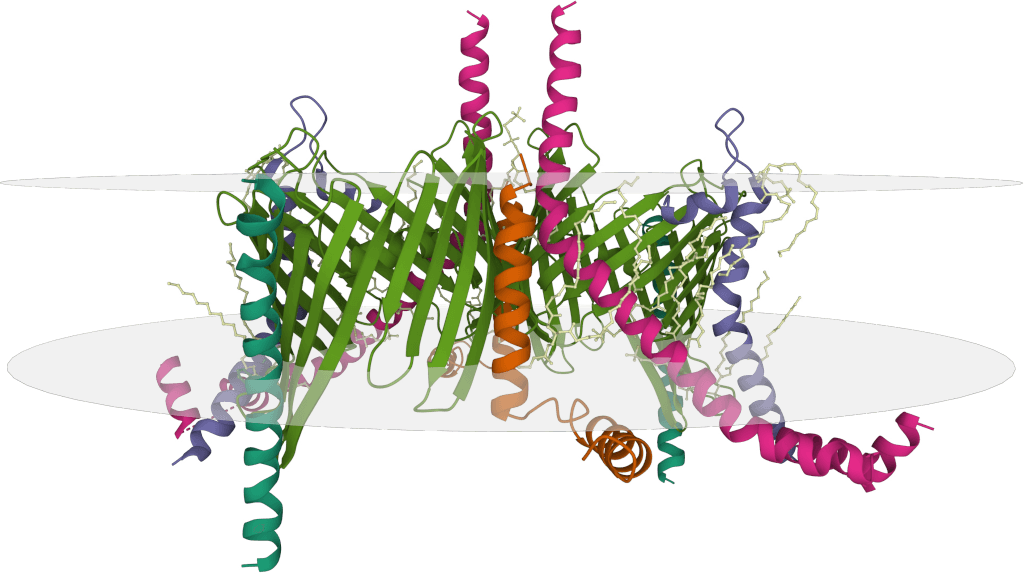
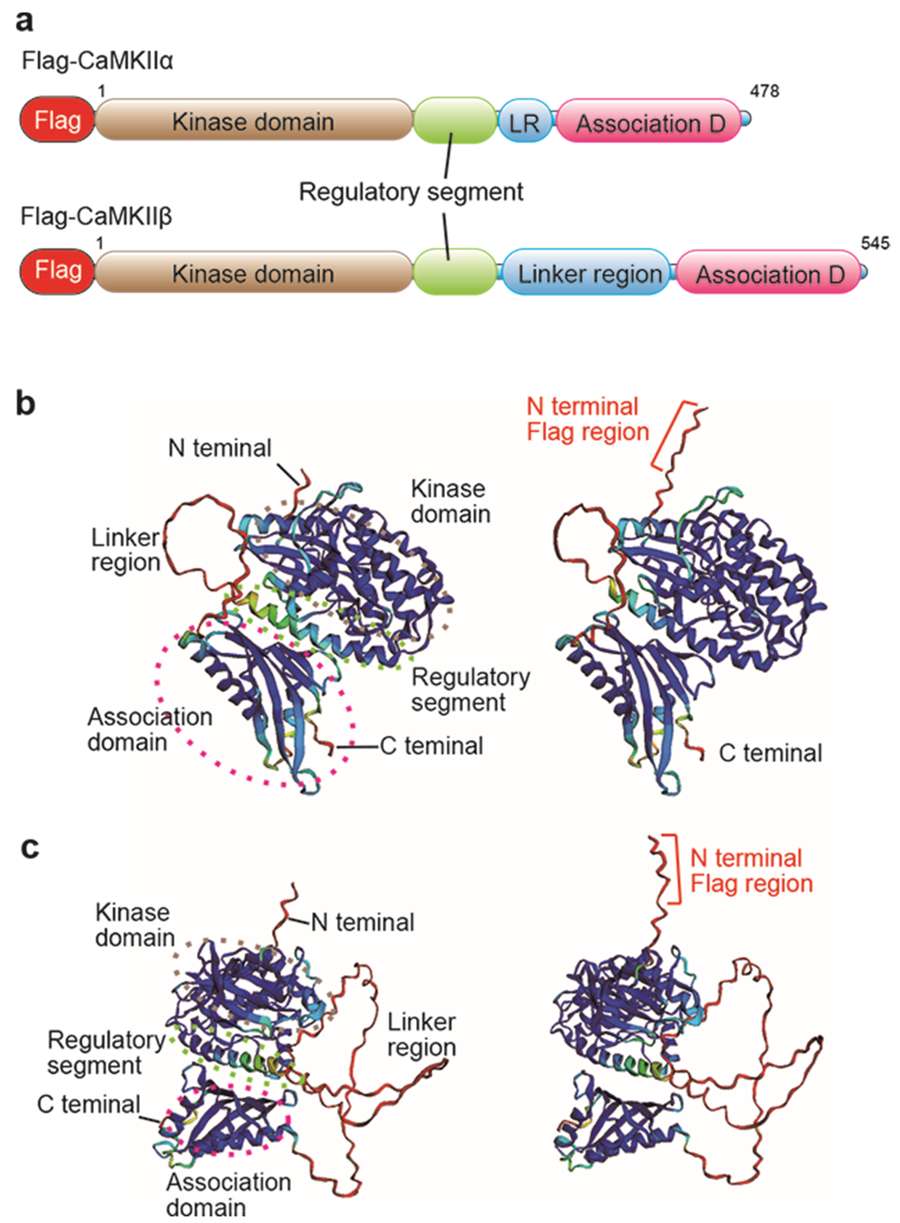 Figure 3. Structural prediction of FLAG-tagged CaMKIIα and CaMKIIβ proteins highlighting domain organization and antibody accessibility. (Aoto K, et al., 2022)
Figure 3. Structural prediction of FLAG-tagged CaMKIIα and CaMKIIβ proteins highlighting domain organization and antibody accessibility. (Aoto K, et al., 2022)
Epitope Tagging Techniques
Epitope Tagging Mechanism
Epitope tags are introduced through genetic engineering techniques. The tag's sequence is inserted into an expression vector containing the gene of interest. Tags can be fused to either end of the protein (N-terminal or C-terminal), depending on the desired experimental context. Detection is typically accomplished using specific monoclonal or polyclonal antibodies.
Molecular Biology Approaches
Several molecular biology techniques are employed to introduce these tags:
- PCR Cloning: This method uses polymerase chain reaction (PCR) to amplify the gene of interest with primers that incorporate the epitope tag sequence. The amplified product is then inserted into an expression vector, ensuring in-frame fusion of the tag to the protein.
- Expression Vectors: Plasmids designed for protein expression in various systems (e.g., E. coil, yeast, mammalian cells) often contain multiple cloning sites and promoters suitable for high-level expression. These vectors can be engineered to include epitope tags at the N- or C-terminus of the protein.
- Recombinase-Mediated Tagging: Site-specific recombinases, such as Cre or FLP, facilitate the insertion of epitope tags into specific genomic locations. This approach allows for controlled expression of tagged proteins under native regulatory elements.
- CRISPR/Cas9 Genome Editing: The CRISPR/Cas9 system enables precise insertion of epitope tags into endogenous gene loci. By designing guide RNAs targeting the desired insertion site and providing a donor template with the tag sequence flanked by homology arms, researchers can achieve efficient knock-in of tags. Studies have demonstrated knock-in efficiencies ranging from 5% to 30% in various cell types without the need for selection markers.
Considerations for Effective Tagging
The success of epitope tagging depends on several factors that influence the expression, stability, and functionality of the tagged protein:
- Tag Positioning: The choice between N-terminal and C-terminal tagging can significantly impact protein function. For instance, tagging the N-terminus may interfere with signal peptides or localization sequences, while C-terminal tags might affect protein folding or stability. Empirical testing is often necessary to determine the optimal tag position for each protein.
- Flexible Linkers: Incorporating flexible linker sequences between the protein and the tag can reduce steric hindrance and preserve protein function. Commonly used linkers consist of glycine and serine residues, such as the (Gly₄Ser)ₙ motif, which provides flexibility and minimizes interference with protein folding.
- Cleavable Sites: Including protease recognition sites, such as those for TEV (Tobacco Etch Virus) protease, allows for the removal of the tag after purification. This is particularly important when the tag might affect downstream applications or protein activity. TEV protease recognizes the ENLYFQ↓G sequence and cleaves between the Q and G residues. Refer to our article Tag Removing and Protease for more details.
- Pilot Studies: Conducting preliminary experiments to assess the expression, localization, and activity of the tagged protein is crucial. These studies help identify any adverse effects of the tag and optimize conditions for large-scale experiments.
Epitope tagging offers a powerful and flexible approach for studying proteins across diverse biological systems. From detection to purification and interaction mapping, the right tag can dramatically improve your research outcomes. At Creative Biostructure, we combine deep expertise in protein engineering and structural biology to deliver high-quality epitope tagging solutions tailored to your research needs. Our services go beyond basic tagging—we design and validate strategies that ensure optimal protein expression, purification, and downstream functional analysis. Contact us for expert support in accelerating your protein research with precision and confidence.
References
- Brizzard B. Epitope tagging. Biotechniques. 2008, 44(5): 693-695. https://doi.org/10.2144/000112841
- Kimple M E, Brill A L, Pasker R L. Overview of affinity tags for protein purification. Current protocols in protein science. 2013, 73(1): 9.9. 1-9.9. 23. https://doi.org/10.1002/0471140864.ps0909s73
- Song J, Hao Y, Du Z, et al. Identifying novel protein complexes in cancer cells using epitope-tagging of endogenous human genes and affinity-purification mass spectrometry. Journal of proteome research. 2012, 11(12): 5630-5641. https://doi.org/10.1021/pr300598t
- Krachmarova E, Tileva M, Lilkova E, et al. His‐FLAG Tag as a Fusion Partner of Glycosylated Human Interferon‐Gamma and Its Mutant: Gain or Loss?. BioMed Research International. 2017, 2017(1): 3018608. https://doi.org/10.1155/2017/3018608
- Aoto K, Takabayashi S, Mutoh H, et al. Generation of flag/DYKDDDDK epitope tag knock-in mice using i-GONAD enables detection of endogenous CaMKIIα and β proteins. International Journal of Molecular Sciences. 2022, 23(19): 11915. https://doi.org/10.3390/ijms231911915
- Jung J W, Park P G, Lee W K, et al. Production of plant-derived japanese encephalitis virus multi-epitope peptide in Nicotiana benthamiana and immunological response in mice. International journal of molecular sciences. 2023, 24(14): 11643. https://doi.org/10.3390/ijms241411643