Fluorescence Resonance Energy Transfer (FRET) Assay
Membrane proteins account for 1/4-1/3 of total 30000 proteins that human genome encoded. Membrane proteins play an essential role in various complicated and unique cellular processes including materials transportation, cell recognition, immune response, signal transduction and regulation, and energy transfer, et.al. Almost 70% of the known or investigational drug targets are membrane proteins. It is still a remaining challenge to determine the structures and perform functional assays of membrane proteins.
Creative Biostructure has established an excellent membrane protein gene-to-structure service platform by a group of experienced professionals. Our full-set membrane protein services including expression & purification, crystallization & determination, and various functional analysis both in vivo and in vitro, proceeding your scientific research at an accelerating and exiting pace. Creative Biostructure can design and provide custom Mempro™ Fluorescence Resonance Energy Transfer (FRET) analysis or FRET assay for function research of membrane protein interactions.
Protein-protein interactions are crucial to signaling networks of membrane proteins. However, Fluorescence resonance energy transfer can only take place if the donor-acceptor distance is no more than 10 nm, making it a very powerful tool to detect and determine membrane protein interactions.
Fluorescence resonance energy transfer (FRET) assay, one of our most advanced and desirable method with extensive application range, performs assays to directly detect the oligomerization state and oligomerization degree of membrane proteins in their native environment. FRET is an distance-depended interaction between the fluorescent donor-acceptor pairs in close proximity, in which fluorescence energy is transferred from an excited donor to a suitable acceptor molecule non-radiatively. The efficiency of FRET extremely depends on the donor-acceptor distance and on overlap spectra of donor-emission and acceptor-excitation.
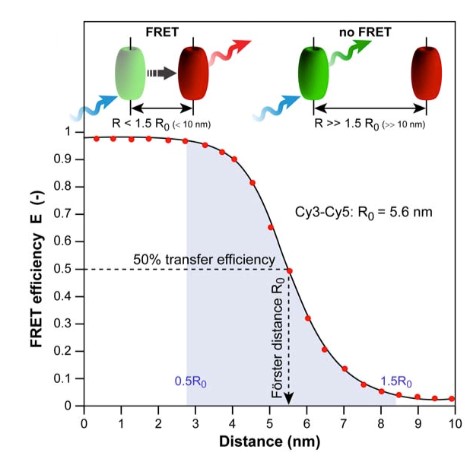 Figure 1. Schematic graph of a
photophysical process-FRET (Molecules, 2012)
Figure 1. Schematic graph of a
photophysical process-FRET (Molecules, 2012)
FRET can only take place if the donor-acceptor distance is no more than 10 nm, making it a very powerful tool to detect and determine membrane protein interactions. Creative Biostructure can provide Mempro™ FRET platform to perform custom membrane protein structural and functional analysis.
•Mempro™ FRET with custom donor-acceptor pair
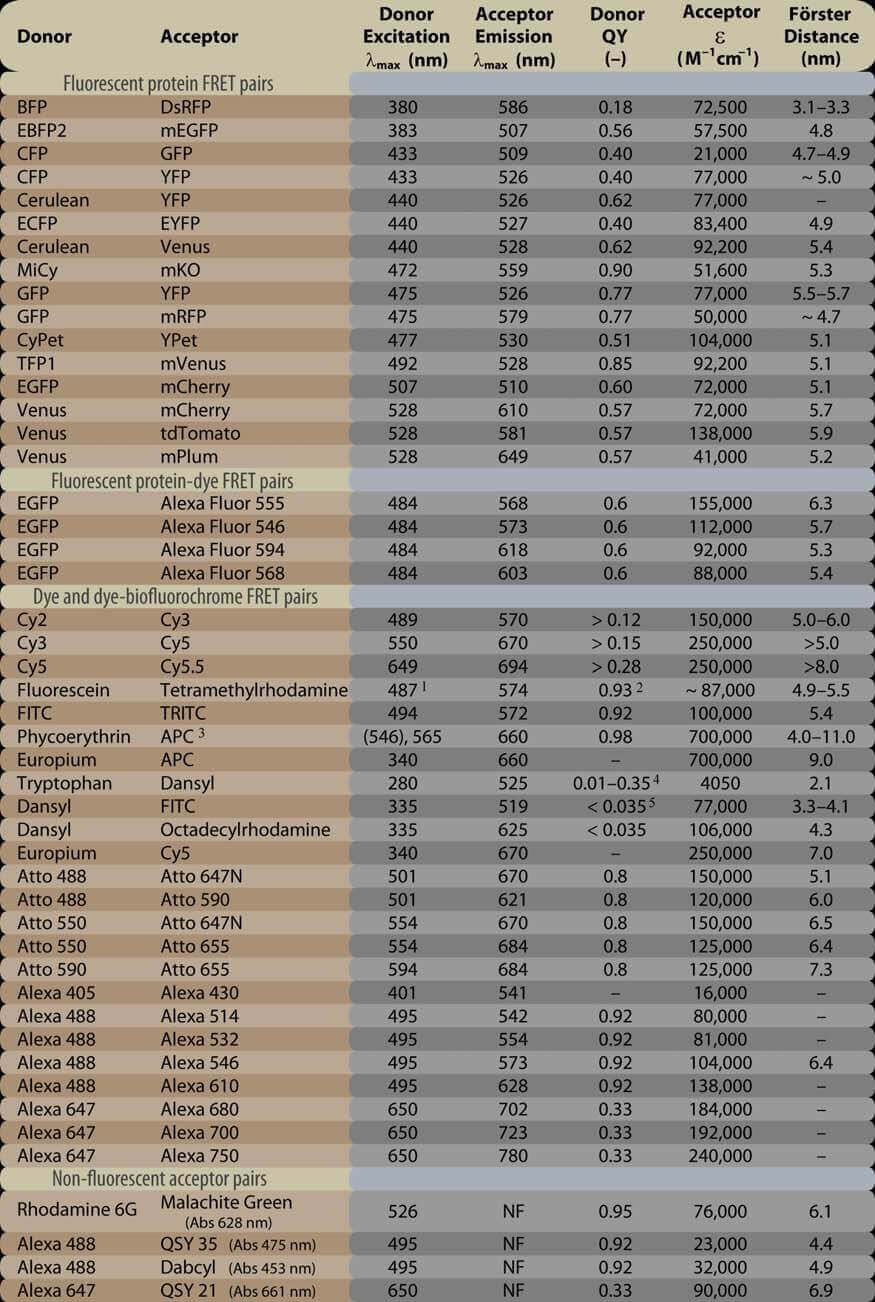
Take the great influence of Forster Distance on FRET into consideration, Creative Biostructure can help you to choose the optimal fluorescent donor-acceptor pair according to your specific membrane protein research requirement.
Table 1. Popular FRET donor-acceptor pairs and their relevant photophysical properties.
Optimal Conditions for FRET:
1. Donor-acceptor pair must be in close distance (typically 1–10 nm).
2. Overlap between acceptor absorption spectrum
and donor emission spectrum.
3. Orientations between donor and acceptor must be approximately parallel.
•Mempro™ FRET aiming at custom purposes
FRET can provide not only qualitative measurements but also quantitate data in membrane function studies. Creative Biostructure has developed a full set of
FRET methods such as 1) Upconversion FRET, 2) Photochromic FRET,
3) Single-Molecule-FRET, and 4) FRET Frustration et.al. Creative Biostructure is your competent and professional
scientific research parter to fulfill all kinds of FRET applications of membrane proteins, including:
1. Structure and conformation of membrane
proteins,
2. Spatial distribution of membrane proteins,
3. Oligomerization of membrane protein complexes,
4. Membrane protein involved
receptor/ligand interactions,
5. Interaction between membrane lipids and membrane proteins.
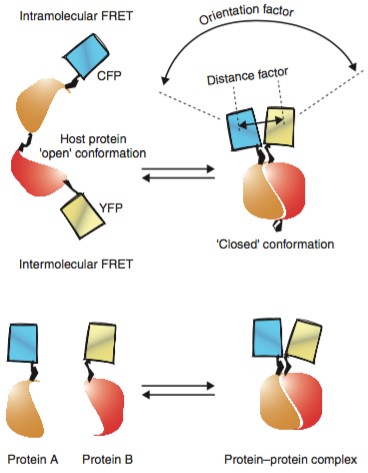 Figure 2. Intramolecular and
intermolecular FRET (Current Opinion in Structural Biology, 2001)
Figure 2. Intramolecular and
intermolecular FRET (Current Opinion in Structural Biology, 2001)
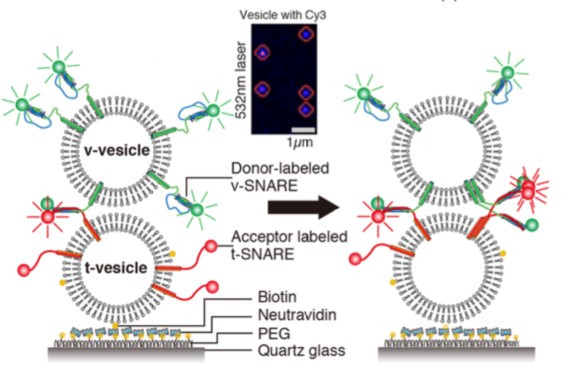 Figure 3. Application of sigle-molecule
FRET (J. Am. Chem. Soc., 2013)
Figure 3. Application of sigle-molecule
FRET (J. Am. Chem. Soc., 2013)
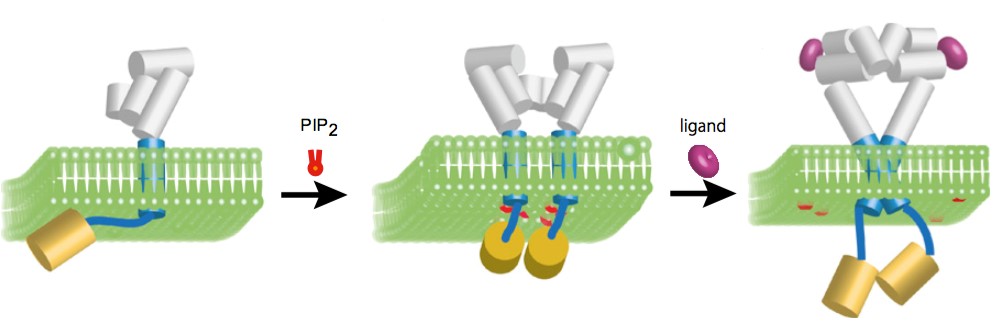 Figure 4. Measuring interaction between
membrane proteins and both lipids and ligands by FRET (PNAS, 2013)
Figure 4. Measuring interaction between
membrane proteins and both lipids and ligands by FRET (PNAS, 2013)
•Mempro™ FRET with custom imaging approaches
Creative Biostructure developed a series of techniques to determine FRET. We generally provide three custom approaches have proven to be particularly useful
based on practical considerations:
1. Donor and Acceptor Photobleaching
FRET can be built up by accessing the bleaching rate of
the donor with and without the presence of the acceptor. The mainly two advantages of this approach are: relatively straightforward, easy to carried out.
Appropriate filter sets and a powerful light source that enable to bleach the acceptor are required.
2. Sensitized Emission
Sensitized emission is the simplest method to detect FRET, and the most ideal condition for this method is that the channels of donor and acceptor
would be completely separated and there is no cross talk between them.
3. Fluorescence Lifetime Imaging Microscopy
Fluorescence
Lifetime Imaging Microscopy, also called FLIM, can be used to map the spatial distribution of the fluorochromes lifetimes both within microscopic images and
living cells. Creative Biostructure can determine the accurate spatial location or distribution of membrane proteins with high resolution and specificity in
living cells.
Creative Biostructure also provide an array of Mempro™ functional assays services. Please feel free to contact us for a detailed quote.
Ordering Process
References:
H. C. Ishikawa-Ankerhold, et al. (2012). Advanced Fluorescence Microscopy Techniques-FRAP, FLIP, FLAP, FRET and FLIM. Molecules,
17(3): 4047-4132.
K. Truong and M. Ikura. (2001). The use of FRET imaging microscopy to detect protein–protein interactions and protein conformational changes in vivo.
Current Opinion in Structural Biology, 11: 573-578.
W. Bae, et al. (2013). Real-Time Observation of Multiple-Protein Complex Formation with Single-Molecule FRET. J. Am. Chem. Soc.,
135(28): 10254-10257.
C. Matsushita, et al. (2013). Transmembrane helix orientation influences membrane binding of the intracellular juxtamembrane domain in Neu receptor
peptides. Proc. Natl. Acad. Sci. USA, 110(5): 1646–1651.
