Creative Biostructure is confident to provide the superb services to produce high-quality voltage-gated K+ channel accessory protein (MinK) in its native state based on our experienced scientists and advanced Mempro™ cell-based expression system.
Voltage-gated K+ channels are specific transmembrane channels for K+, which are sensitive to the voltage changes through cell membranes. Voltage-gated K+ channel accessory protein, also known as MinK or KCNE1, is one of the KCNE family which include five β subunits of voltage-gated K+ channel. In the transmembrane domain of MinK, there are some residues in close distance to the KCNQ1 selectivity filter. The C-terminal domain of MinK (especially residue 73-79) is required for stimulation of slow delayed K+ current. MinK plays a pivot role of modulating the cardiac and epithelial voltage-gated K+ channel α subunit, KCNQ1. Mutations disrupted function of MinK result in Long QT syndrome, while mutations gain function of MinK lead to early-onset atrial fibrillation. Those clinical significance of MinK make this membrane protein a prospective drug target in recent studies.
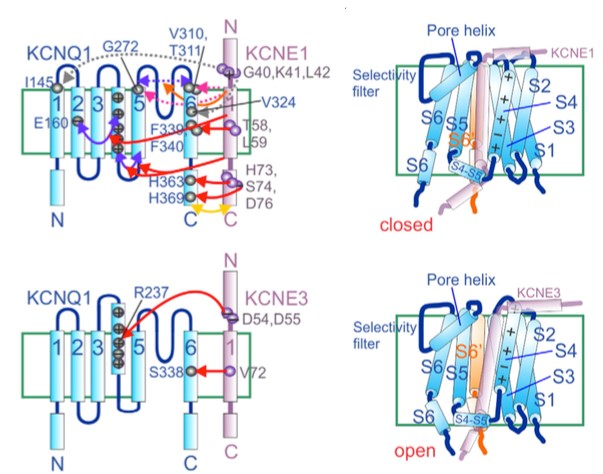
Figure 1. Mechanisms of KCNQ1 gating regulated by MinK and KCNE3. (Gene,2016)
Creative Biostructure is your No.1 choice to produce high-yiled membrane proteins by cell-based expression systems, we can provide various strategies for Mempro™ cell-based voltage-gated K+ channel accessory protein (MinK) production, including:
• Mempro™ Voltage-gated K+ Channel Accessory Protein (MinK) Production in Insect Cells System
Insect cells such as Sf9, Sf21 and High Five, are major used for eukaryotic membrane protein expression. Mempro™ protein expression by insect cells system assures you the enhancement of expression and reduction of the truncated proteins, and easy to perform scale up the high-yield production.
• Mempro™ Voltage-gated K+ Channel Accessory Protein (MinK) Production in Mammalian Cells System
Creative Biostructure can provide Mempro™ protein production in mammalian cells system. Cell lines derived from HeLa, COS, BHK-21, CHO, and HEK293 are widely used to express membrane proteins. This system helps the membrane proteins to perform correct folding and post-translational modifications and maintain the best structural and functional features.
• Mempro™ Voltage-gated K+ Channel Accessory Protein (MinK) Production in Bacterial Cells System
Escherichia coli (E. coli) is the most powerful bacterial host for membrane protein production, providing multiple options for the optimization of the production protocols. Whole-cell fluorescence can be used to monitor the membrane protein-GFP fusions production by our optimized Lemo21 (DE3) host, which enables the integrity of the production.
• Mempro™ Voltage-gated K+ Channel Accessory Protein (MinK) Production in Yeast Cells System
Yeast cells (such as saccharomyces cerevisiae and pichia pastoris), are perfect eukaryotic hosts with following advantages: high cell densities, fast growth rates, economic media.
Creative Biostructure has solved series of the membrane proteins production barriers by optimizing expression vectors, host strains, growth conditions, co-expression of helper proteins and fusion tags, etc, and we are able to provide you the high-quality voltage-gated K+ channel accessory protein (MinK) production service.
We offer various Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
G. W. Abbott. (2016). KCNE1 and KCNE3: The yin and yang of voltage-gated K+ channel regulation. Gene, 576: 1-13.
G. W. Abbott. (2013). KCNE genetics and pharmacogenomics in cardiac arrhythmias: much ado about nothing? Expert. Rev. Clin. Pharmacol., 6(1), 49–60.
Y. Gofman, et al. (2012). How does KCNE1 regulate the Kv7.1 potassium channel? Model-structure, mutations, and dynamics of the Kv7.1- KCNE1 complex. Structure 20 (8), 1343–1352.
Y. Li, et al. (2011). KCNE1 enhances phosphatidylinositol 4,5-bisphosphate (PIP2) sensitivity of IKs to modulate channel activity. Proc. Natl. Acad. Sci. U. S. A., 108(22), 9095–9100.
E. Schulze-Bahr, et al. (1997). KCNE1 mutations cause Jervell and Lange–Nielsen syn- drome. Nat. Genet. 17(3), 267–268.
