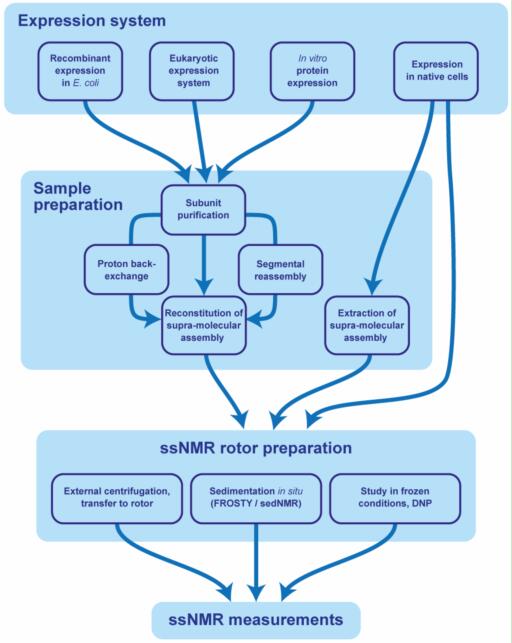
In materials science, solid-state chemistry, and pharmaceutical research, understanding the structural and dynamic properties of crystalline materials is crucial. Techniques like X-ray diffraction (XRD) and electron microscopy provide long-range order information and atomic positioning, but they often fall short in revealing local environments, dynamic processes, or amorphous components. This is where solid-state Nuclear Magnetic Resonance (NMR) steps in as a unique and powerful technique.
Solid-state NMR, unlike its solution counterpart, is adept at probing atomic-scale environments without the need for long-range order or sample solubility. It offers atom-specific, non-destructive, and orientation-sensitive data, making it indispensable for studying both perfect crystals and real-world, complex materials riddled with defects, disorder, and interfaces.
Creative Biostructure offers advanced solid-state NMR services for investigating chemical structures, spatial arrangements, and conformations across a wide range of substances—including organic and inorganic compounds, polymers, natural products, and small proteins.
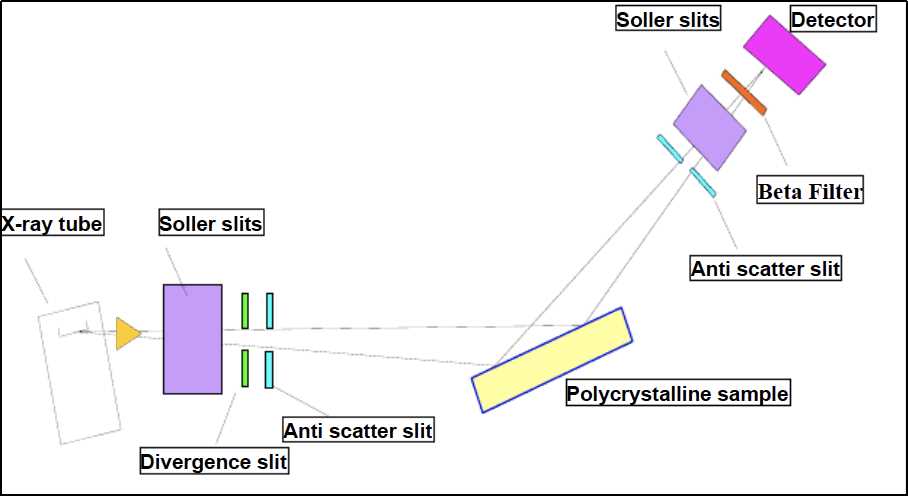
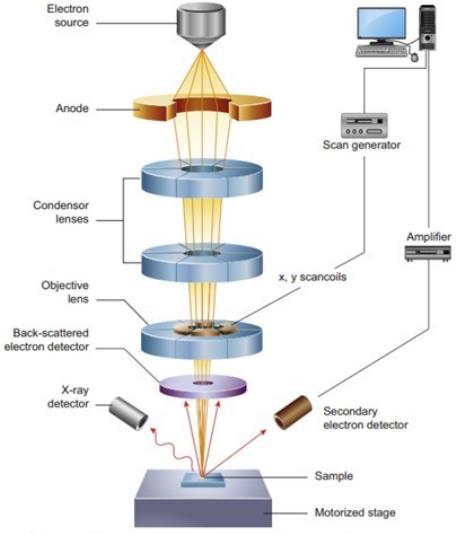
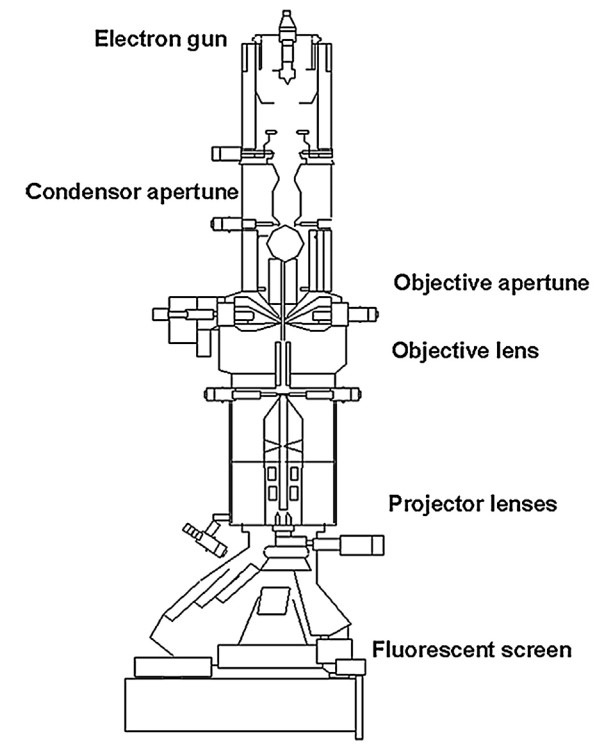
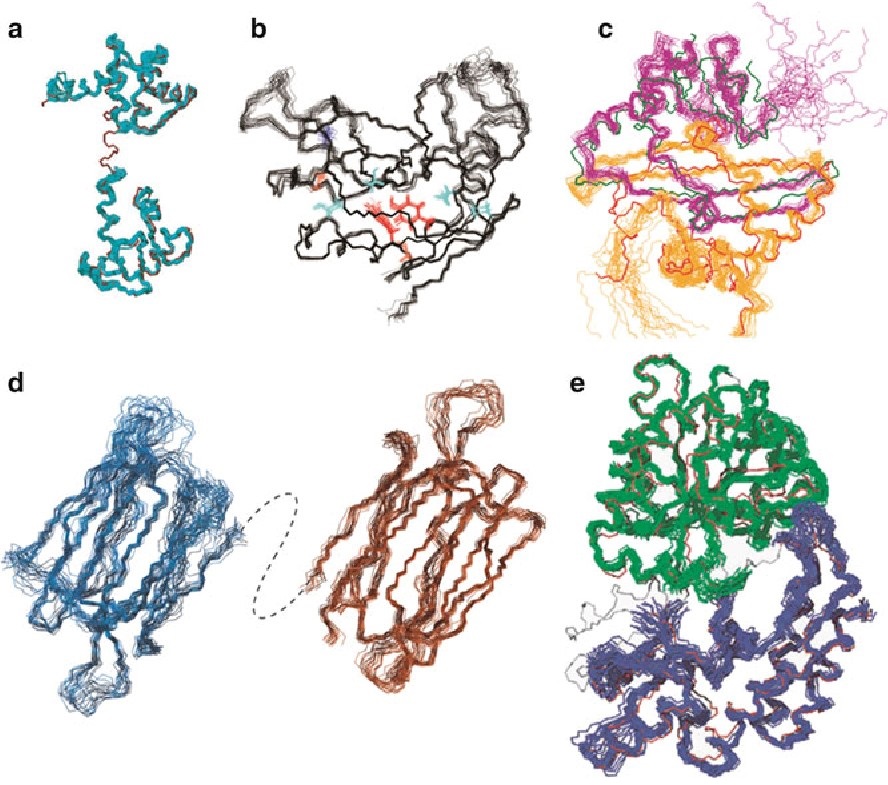
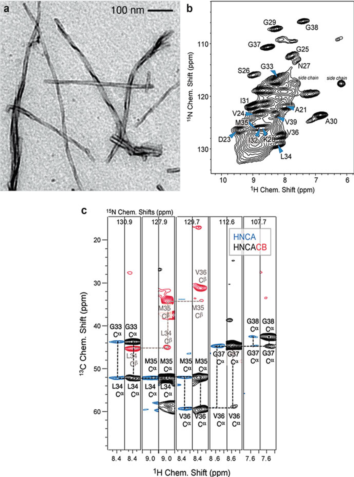
 Figure 1. Solid-state NMR spectroscopy for studying crystalline microporous materials. (Ashbrook et al., 2014)
Figure 1. Solid-state NMR spectroscopy for studying crystalline microporous materials. (Ashbrook et al., 2014)
Basic Principles of NMR Spectroscopy
At the heart of NMR spectroscopy lies the principle of nuclear spin. Certain isotopes—such as 1H, 13C, 15N, 19F, 29Si, and 31P—possess a non-zero nuclear spin, making them responsive to an external magnetic field. When subjected to such a field, these nuclei occupy discrete energy levels. Radiofrequency (RF) pulses excite these nuclei, and the subsequent relaxation emits signals characteristic of the local atomic environment.
Key concepts include:
- Chemical shift (δ): Measures the shielding of nuclei by surrounding electrons, reflecting local electronic environments.
- J-coupling: Scalar interaction between spins through chemical bonds (typically less dominant in solids).
- Dipolar coupling: Magnetic interaction between two nuclei; highly prominent in solids.
- Quadrupolar interaction: Relevant for nuclei with spin > 1/2 (e.g., 27Al, 17O), due to electric field gradients around the nucleus.
Principles Specific to Solid-State NMR
To overcome the static, anisotropic interactions present in solids, solid-state NMR employs a range of physical and computational techniques:
- Magic-Angle Spinning (MAS): The sample is spun rapidly (~10–100 kHz) at 54.74° relative to the magnetic field. This averages out anisotropic components of interactions, narrowing lines dramatically.
- Cross-Polarization (CP): Enhances the signal of low-gamma nuclei (e.g., 13C, 15N) by transferring polarization from abundant 1H nuclei.
- High-Power Decoupling: Continuous RF irradiation suppresses heteronuclear dipolar couplings during acquisition, improving resolution.
- Multiple Pulse Sequences: Used to selectively probe particular interactions (e.g., REDOR, CP-MAS, HETCOR).
These methods allow solid-state NMR to resolve structural details otherwise inaccessible in static solid samples.
Techniques to Improve Resolution in Solid-State NMR
Beyond MAS and CP, several innovations help maximize spectral resolution and sensitivity:
- High-Field Magnets: Higher magnetic fields increase resolution and reduce quadrupolar broadening, improving spectral dispersion.
- Ultra-Fast MAS: Spinning rates exceeding 60 kHz improve resolution, especially in proton-detected experiments.
- Isotope Enrichment: Enriching samples with 13C, 15N, or 17O increases sensitivity and allows for complex correlation experiments.
- Dynamic Nuclear Polarization (DNP): Transfers polarization from electrons to nuclei, resulting in signal enhancements up to 100-fold.
- 2D Solid-State NMR: Techniques such as 1H–13C HETCOR, 13C–13C DARR, and 27Al MQMAS provide spatial and bonding correlations.
Each method is tailored to the nucleus of interest, sample characteristics, and specific analytical goals.
Select Service
- Materials Science
- Medical and Pharmaceutical Science
- Solid-state NMR Services
- X-ray powder diffraction (XRPD) Analysis
- X-ray Crystallography Services
- Cryo-Electron Microscopy (Cryo-EM) Services
- Solution-state NMR
- Polymer Materials Analysis
- Natural Product Analysis
- Membrane Protein Structure Determination by Solid-state NMR
- Quadrupole Nuclei in Inorganic Materials
- NMR of Quadrupole Nuclei in Organic Compounds
- Scanning Electron Microscope Service
- Transmission Electron Microscope Service
- Magic-Angle Spinning (MAS) Solid-state NMR Service
- Stable-Isotope Aided NMR Service
Related Reading
- What is Solid-State NMR and How Is It Used?
- Overview of Powder X-ray Diffraction (PXRD)
- What Is Single-Crystal X-ray Diffraction (XRD) and How Does It Work?
- Cryo-Electron Tomography (Cryo-ET) Technology
- Transmission Electron Microscopy vs. Scanning Electron Microscopy
- Latest Advancements in NMR Technology
Application of Solid-State NMR to Crystalline Materials
Structure Elucidation
Solid-state NMR is a powerful tool for elucidating the structures of crystalline materials. Unlike X-ray diffraction which reports on the electron-density distribution, NMR spectroscopy probes the nuclei themselves and provides detailed information about the local atomic environment. For example, it can reveal the positions and interactions of hydrogen atoms, which are often difficult to determine accurately using X-ray diffraction alone. Moreover, advanced techniques such as Dynamic Nuclear Polarization (DNP)-enhanced solid-state NMR can reduce experimental time and allow the study of more complex structures.
Polymorphism Detection
Polymorphism, the existence of a compound in different crystal forms, is an important phenomenon in many fields. Solid-state NMR can effectively detect and differentiate polymorphs. Different polymorphs have distinct NMR spectra due to their different molecular conformations and packing arrangements. For example, 13C solid-state NMR spectroscopy has been used to identify and characterize various polymorphs of pharmaceutical compounds, providing valuable information for drug development.
Hydrogen Bonding and Molecular Dynamics
Hydrogen bonding plays a crucial role in the structure and properties of many crystalline materials. Solid-state NMR is particularly useful in studying hydrogen bonds because it can directly detect hydrogen atoms and their interactions. Additionally, solid-state NMR can probe molecular dynamics in crystalline materials over a wide range of timescales, from seconds to picoseconds. This enables researchers to investigate processes such as molecular rotation, diffusion, and reorientation, which are important for understanding the material's properties and behavior.
Defect and Disorder Analysis
Defects and disorder in crystalline materials can significantly affect their properties. Solid-state NMR can provide detailed information about the types, concentrations, and locations of defects and disorder in the crystal lattice. For example, it can detect vacancies, interstitial atoms, and dislocations, and study their interactions with the surrounding atoms. This information is crucial for understanding the material's mechanical, electrical, and thermal properties.
Crystallinity Assessment
Solid-state NMR can be used to assess the degree of crystallinity in materials. It can distinguish between crystalline and amorphous regions, and quantify the proportion of each phase. This is particularly useful for studying materials that have a mixture of crystalline and amorphous components, such as polymer blends and composites.
Case Studies
Case 1: Revealing hidden carbamazepine conformations via 2D 1H-15N HMBC NMR
Two-dimensional 1H–15N HMBC NMR spectra of the well-known anticonvulsant carbamazepine, recorded in various organic solvents, reveal the presence of hidden conformers in saturated solutions. The observed distribution of these conformers is attributed to the presence of a solid phase within the saturated system. Additionally, a subtle influence of ring currents on different molecular conformations was detected, offering a straightforward method for identifying otherwise undetectable conformers. These hidden conformers were identified in three solvents: dimethyl sulfoxide, chloroform, and dichloromethane.
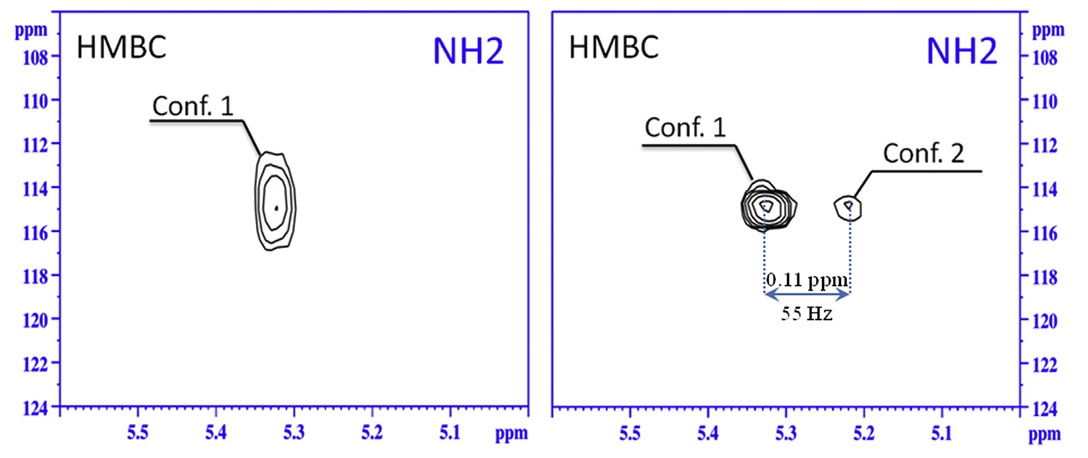 Figure 2. 1H-15N HMBC spectra of carbamazepine in (left) a solution without solid phase and (right) a solution with solid phase in chloroform, obtained at the natural isotope abundance (300 K, 500 MHz, Bruker Avance III). (Khodov et al., 2021)
Figure 2. 1H-15N HMBC spectra of carbamazepine in (left) a solution without solid phase and (right) a solution with solid phase in chloroform, obtained at the natural isotope abundance (300 K, 500 MHz, Bruker Avance III). (Khodov et al., 2021)
Case 2: Studying distribution of aluminum species in zeolite catalysts with 27Al NMR
This study investigates the structure of aluminum species in H-ZSM-5 zeolite catalysts using high-field 27Al NMR spectroscopy across magnetic field strengths from 7.05 to 35.2 T. The results show that, in dehydrated commercial H-ZSM-5 catalysts (Si/Al = 11.5–40), aluminum exists primarily as framework or partially coordinated framework species. Prior to hydrothermal exposure, non-framework Al species are undetectable. Advanced 2D NMR techniques (27Al MQMAS, 1H–27Al and 29Si–27Al D-HMQC) confirm this finding and clearly distinguish non-framework species generated only after hydrothermal treatment. The study also highlights how hydration affects spectral interpretation, and emphasizes that accurate characterization—especially for high-Al content catalysts—requires data from multiple magnetic field strengths. These insights enable better prediction of catalyst behavior under operating conditions and help differentiate contributions from various hydroxyl groups.
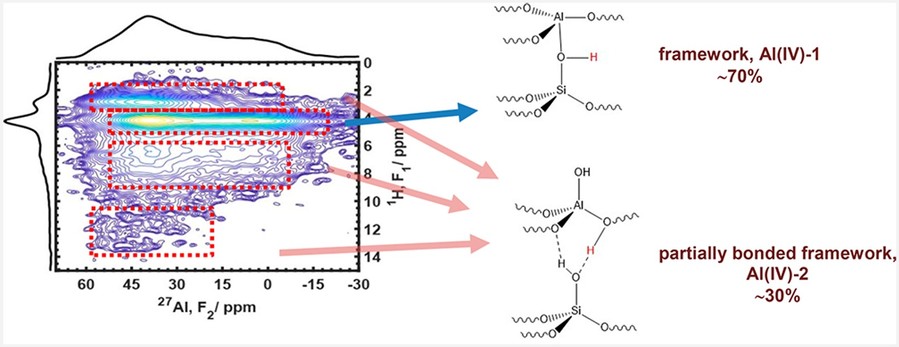 Figure 3. Multi-field 27Al NMR combined with 2D correlation techniques reveals aluminum speciation and in in zeolite catalysts. (Chen et al., 2021)
Figure 3. Multi-field 27Al NMR combined with 2D correlation techniques reveals aluminum speciation and in in zeolite catalysts. (Chen et al., 2021)
Case 3: 6Li{7Li} REDOR NMR for evaluating lithium ion conductivity
This study investigates lithium-ion dynamics in garnet-like solid-state electrolytes Li6BaLa2Nb2O12 (M = Ta, Nb) using 6Li{7Li} REDOR NMR. By analyzing temperature-dependent REDOR slopes, the researchers assess Li+ hopping rates across 247–350 K. The Nb-based phase exhibits higher ionic conductivity and a greater activation energy for ion hopping than the Ta-based analog. The findings demonstrate that REDOR NMR is an effective method for comparing Li+ ion dynamics in structurally similar solid-state electrolytes.
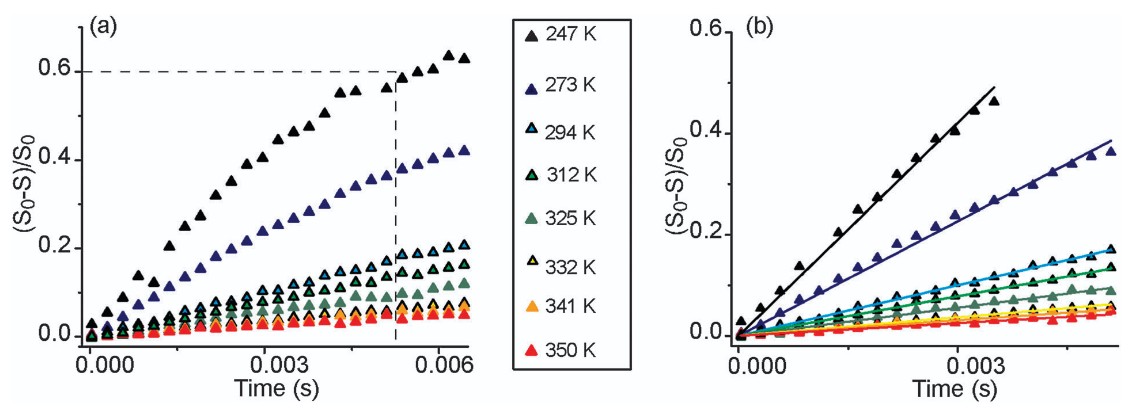 Figure 4. (a) 6Li{7Li}-REDOR curves of Li6BaLa2Nb2O12 over the temperature range of 247 K, where the sample is immobile, to 350 K. (b) Lines of best fit for each temperature have slopes P. (Spencer et al., 2014)
Figure 4. (a) 6Li{7Li}-REDOR curves of Li6BaLa2Nb2O12 over the temperature range of 247 K, where the sample is immobile, to 350 K. (b) Lines of best fit for each temperature have slopes P. (Spencer et al., 2014)
In summary, solid-state NMR spectroscopy is a cornerstone analytical technique for the study of crystalline materials. Its ability to provide atom-specific, local information—without requiring sample solubility or long-range order — makes it uniquely powerful. From identifying polymorphs in pharmaceuticals to probing defects in catalysts and structures in complex frameworks, solid-state NMR delivers where other methods falter. Its integration with modern computational techniques and hardware innovations continues to expand its reach and impact.
With a full-featured NMR platform, Creative Biostructure delivers customized solutions for your crystal material research. Contact us today to learn how our NMR expertise can support your next project!
References
- Ashbrook SE, Dawson DM, Seymour VR. Recent developments in solid-state NMR spectroscopy of crystalline microporous materials. Phys Chem Chem Phys. 2014;16(18):8223-8242.
- Chen K, Gan Z, Horstmeier S, White JL. Distribution of aluminum species in zeolite catalysts:27Al NMR of framework, partially-coordinated framework, and non-framework moieties. J Am Chem Soc. 2021;143(17):6669-6680.
- Khodov I, Efimov S, Krestyaninov M, Kiselev M. Exposing hidden conformations of carbamazepine appearing due to interaction with the solid phase by 2D 1H-15N HMBC NMR spectroscopy. Journal of Pharmaceutical Sciences. 2021;110(4):1533-1539.
- Spencer TL, Plagos NW, Brouwer DH, Goward GR. The use of 6Li{7Li}-REDOR NMR spectroscopy to compare the ionic conductivities of solid-state lithium ion electrolytes. Phys Chem Chem Phys. 2014;16(6):2515-2526.