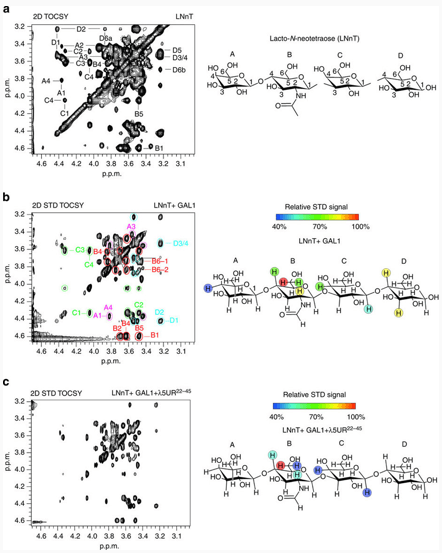
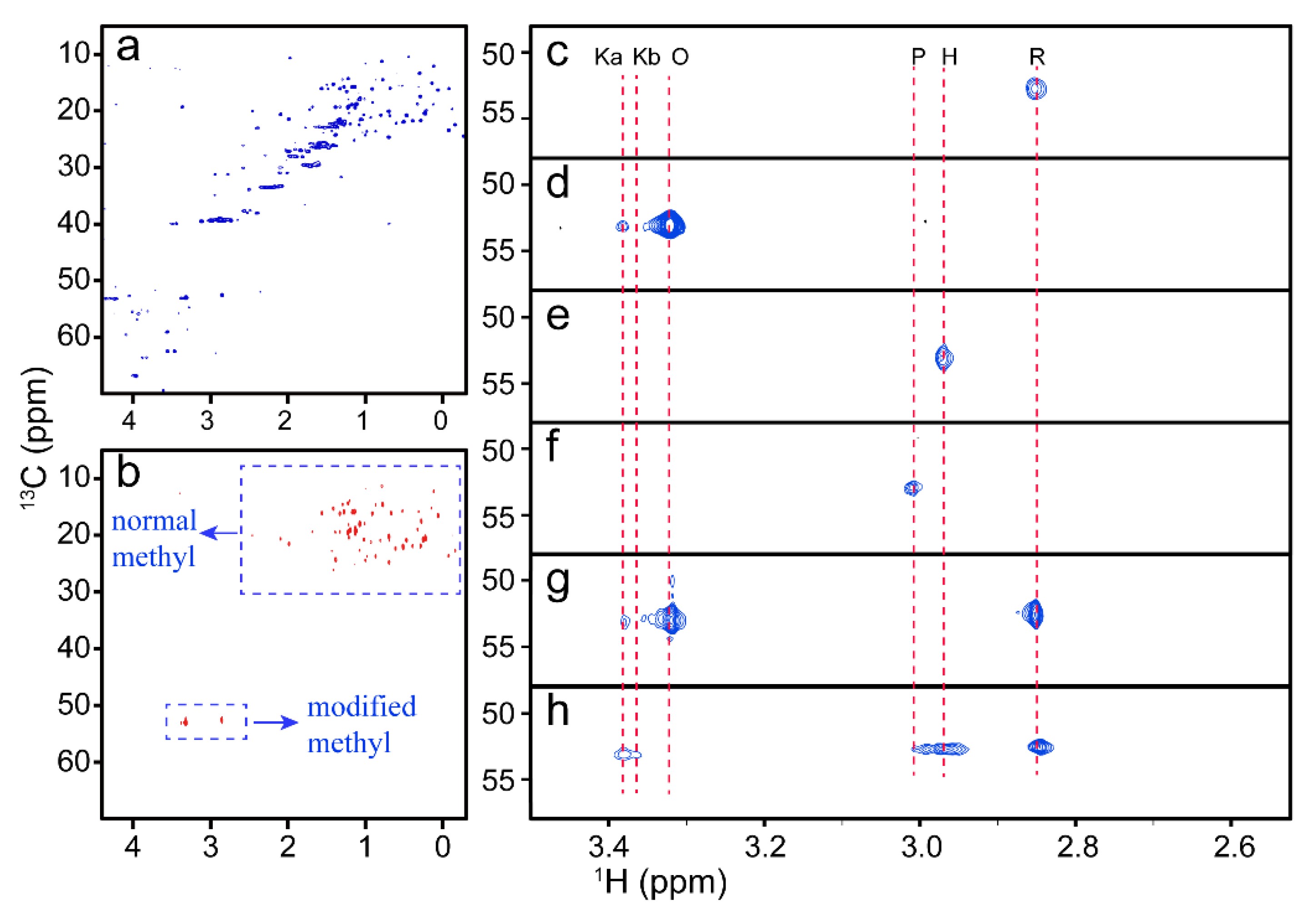
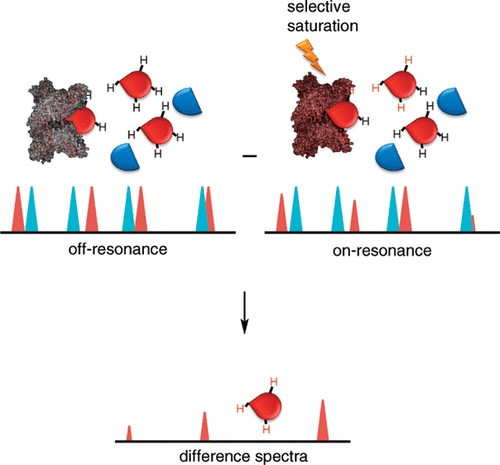
Understanding protein-ligand interactions is critical for elucidating biological mechanisms and guiding drug discovery. Nuclear magnetic resonance (NMR) spectroscopy has emerged as a powerful and versatile technique for studying these interactions in solution, providing atomic-level resolution of dynamic, transient, and weakly interacting complexes. Here, Creative Biostructure provides a comprehensive overview of how NMR is used to study protein-ligand interactions. It includes the underlying principles, types of NMR experiments used, data interpretation strategies, strengths and limitations of the technique, and its applications in structural biology and drug design.
 Figure 1. Crystal structure of W741L mutant androgen receptor ligand-binding domain and (R)-bicalutamide complex. An example of a protein–ligand complex.
Figure 1. Crystal structure of W741L mutant androgen receptor ligand-binding domain and (R)-bicalutamide complex. An example of a protein–ligand complex.
Introduction to Protein-Ligand Interactions
Protein-ligand interactions are fundamental to virtually all biological processes, playing a central role in signaling pathways, enzyme activity, immune responses, and drug action. A ligand—a molecule that binds specifically to a protein-can be a small organic compound, a metal ion, a peptide, or even another protein. The specificity and affinity of these interactions determine cellular function and are key parameters in drug discovery and development. Understanding how ligands associate with their target proteins, the strength and stability of these complexes, and the underlying kinetic and thermodynamic principles provides critical insight into molecular recognition and function.
Techniques such as NMR spectroscopy, X-ray crystallography, surface plasmon resonance (SPR), isothermal titration calorimetry (ITC) are widely used to study these interactions. Among these, NMR offers a unique advantage by allowing real-time analysis of binding events in solution under near-physiological conditions, capturing both structural and dynamic aspects of the interaction. A comprehensive understanding of protein-ligand binding not only advances our knowledge of biochemical mechanisms, but also facilitates the rational design of more effective therapeutic agents.
Basic Principles of NMR
Nuclear Magnetic Resonance
NMR relies on the magnetic properties of certain nuclei, most commonly ¹H, ¹³C, ¹⁵N, and ³¹P. When placed in an external magnetic field, these nuclei absorb and re-emit electromagnetic radiation at characteristic frequencies depending on their chemical environment. The resulting spectra provide detailed information about molecular structure, dynamics, and interactions.
Chemical Shift Perturbation (CSP)
The chemical shift of a nucleus is sensitive to its electronic environment. When a ligand binds to a protein, the chemical environment of nearby nuclei changes, resulting in a chemical shift perturbation. Mapping these shifts allows for the identification of ligand binding sites and understanding the nature of the interaction.
Sample Requirements
To obtain high-quality NMR data, careful attention must be paid to sample preparation. Below are detailed requirements for both protein and ligand samples:
| Protein Requirements | Purity and Solubility | Purity | ≥ 90% (assessed by SDS-PAGE, HPLC, or mass spectrometry) to minimize signal interference from contaminants. |
| Solubility | Minimum concentration of > 0.1 mM (preferably 0.3-1 mM for optimal signal-to-noise ratio). Aggregation should be avoided; dynamic light scattering (DLS) or analytical ultracentrifugation can assess monodispersity. | ||
| Buffer Conditions | Low ionic strength | High salt concentrations can broaden NMR signals (e.g., ≤ 100 mM NaCl). | |
| pH stability | Buffers should maintain a stable pH (e.g., phosphate, Tris, or HEPES) near the pI of the protein to minimize amide exchange broadening. Recommended pH range is 6.0-7.5 (adjust based on protein stability). | ||
| D2O compatibility | Required for locking and shimming; at least 5-10% D2O should be present in the sample. | ||
| Isotopic Labeling (Critical for Larger Proteins) | ¹⁵N-labeling | Enables HSQC experiments for backbone assignment and ligand binding studies. | |
| ¹³C-labeling | Required for triple-resonance experiments (e.g., CBCA(CO)NH, HNCO) for structural determination. | ||
| ²H (Deuterium) labeling | Reduces signal overlap and relaxation effects in proteins > 25 kDa. Fractional deuteration (e.g., 70% D, 30% H) balances sensitivity and resolution. | ||
| Ligand Requirements | Solubility | Must be soluble in the same buffer as the protein (DMSO ≤ 5% if needed). | |
| Non-aggregating | Should not form micelles or precipitates (check via dynamic light scattering or NMR linewidth). | ||
| Concentration range for titrations | Typically, 0.1–10 mM stock solutions. Molar ratios of 1:0.5 to 1:10 (protein:ligand) for binding affinity measurements (e.g., Kₐ, Kₑ). | ||
| Additional Considerations | Reducing agents (e.g., DTT, TCEP) | May be needed to prevent oxidation but should be kept at low concentrations (≤ 1 mM) to avoid signal interference. | |
| Temperature control: | Some proteins require low temperatures (4-25°C) to maintain stability during NMR experiments. | ||
| Protease inhibitors | Necessary for unstable proteins to prevent degradation over long acquisition times. | ||
NMR Techniques for Studying Protein-Ligand Interactions
NMR methods for studying protein–ligand interactions can be broadly classified into ligand-detected and protein-detected techniques.
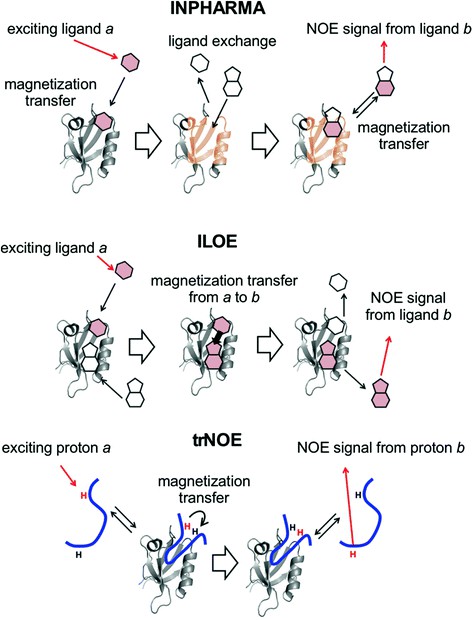
| Ligand-Observed Techniques | Saturation Transfer Difference (STD) NMR | STD NMR selectively saturates protein resonances and transfers this saturation to bound ligands. Only ligands in close contact are saturated, so the signals that appear are indicative of binding. It's excellent for detecting weak interactions and screening libraries. |
| WaterLOGSY (Water–Ligand Observed via Gradient SpectroscopY) | WaterLOGSY exploits the magnetization transfer from water to ligands via protein. Bound ligands show inverted signals compared to unbound ones. It complements STD and is useful for fragment screening. | |
| Protein-Observed Techniques | ¹H-¹⁵N HSQC (Heteronuclear Single Quantum Coherence) | The 2D ¹H-¹⁵N HSQC is a "fingerprint" of the protein backbone amides. Upon ligand binding, residues involved in the interaction exhibit chemical shift perturbations (CSPs). This is a primary tool for mapping binding interfaces and understanding binding modes. |
| ³D Experiments (HNCA, HNCACB, etc.) | For detailed assignments and structural changes, triple resonance experiments are required. They help to trace the entire protein backbone and are crucial when the interaction leads to major conformational changes. | |
| Paramagnetic Relaxation Enhancement (PRE) | Paramagnetic tags on ligands or proteins alter relaxation rates of nearby nuclei. This provides long-range distance constraints for mapping the orientation of bound ligands. | |
| Transferred NOE (trNOE) | For small, fast-exchange ligands, trNOE allows observation of their conformation when bound to the protein. It reveals low-energy bioactive conformers that are essential for structure-based drug design. |
Binding Kinetics and Thermodynamics via NMR
NMR is a powerful tool for characterizing protein-ligand interactions in terms of both thermodynamics and kinetics. Through systematic titration experiments in which increasing concentrations of ligand are added to a protein sample, researchers can monitor changes in the NMR spectra—such as chemical shift perturbations or variations in signal intensity—to determine key parameters. These include the dissociation constant (KD), which reflects binding affinity, and the stoichiometry of the interaction (e.g., 1:1 or 1:2 binding). In addition, depending on the nature of the spectral changes, NMR can reveal valuable information about the rate of exchange between the free and bound states.
Fast, Intermediate, and Slow Exchange
The appearance of NMR signals during titration is governed by the timescale of molecular exchange relative to the NMR timescale, which determines how quickly the ligand and protein switch between free and bound states:
- Fast Exchange: When the exchange is fast, a single peak is observed that gradually shifts in position as the ligand concentration increases. This regime is common for weak to moderate affinity interactions and allows precise mapping of chemical shift changes to estimate KD values.
- Intermediate Exchange: In this regime, the exchange is comparable to the NMR timescale, resulting in broadened or even disappearing peaks. This complicates analysis, but may indicate intermediate binding affinities or transient interactions.
- Slow Exchange: When the exchange is slow, two separate peaks appear—one for the free state and one for the bound state—clearly indicating the coexistence of the two populations. This regime is typically seen in strong binding scenarios and allows direct integration of peak intensities to calculate KD.
NMR Line Broadening
Ligand binding can also lead to NMR line broadening, an effect caused by increased molecular size (and thus slower tumbling) or conformational exchange within the complex. When a small ligand binds to a large protein, its rotational correlation time increases, leading to broader lines in the spectrum. In addition, if binding induces conformational flexibility or dynamic exchange between states, further line broadening may occur. Monitoring these changes provides qualitative, and in some cases semi-quantitative, insight into both the strength of the binding and the structural dynamics of the interaction. This makes line broadening a useful, albeit indirect, indicator of binding events, especially when full chemical shift assignment is not possible.
Applications in Drug Discovery and Structural Biology
- Fragment-Based Drug Discovery (FBDD): NMR is extensively used in FBDD, where small fragments (MW < 300 Da) are screened for weak binding and then optimized. STD, WaterLOGSY, and CSPs help identify hits and guide chemical elaboration.
- Epitope Mapping: NMR reveals which parts of the ligand are involved in binding, guiding medicinal chemistry efforts. STD NMR and trNOE are especially useful for mapping ligand epitopes.
- Structure Determination of Complexes: Using CSPs and distance constraints (NOEs, PREs), NMR can be used to model or refine the 3D structure of protein–ligand complexes in solution, especially when crystallography is not feasible.
- Allosteric Binding and Conformational Changes: NMR is sensitive to changes even distant from the binding site. This makes it ideal for detecting allosteric effects, conformational changes, or cryptic binding pockets.
Select Service
Case Studies
Case 1: Ligand-based competition binding by real-time 19F NMR in human cells
This study presents a novel real-time in-cell ¹⁹F NMR approach for measuring ligand binding affinities directly in living human cells. Traditional in-cell NMR methods face challenges such as target invisibility or background noise from cellular components, especially when using ¹H signals. By using fluorinated ligands and detecting them with ¹⁹F NMR, researchers can circumvent these issues. The method enables competitive binding assays in cells using a fluorinated reference compound and has been successfully used to evaluate compound interactions with Hsp90α, a key protein target. This technique has broad potential to improve early-stage drug design by providing direct insight into ligand-target interactions in their native cellular environment.
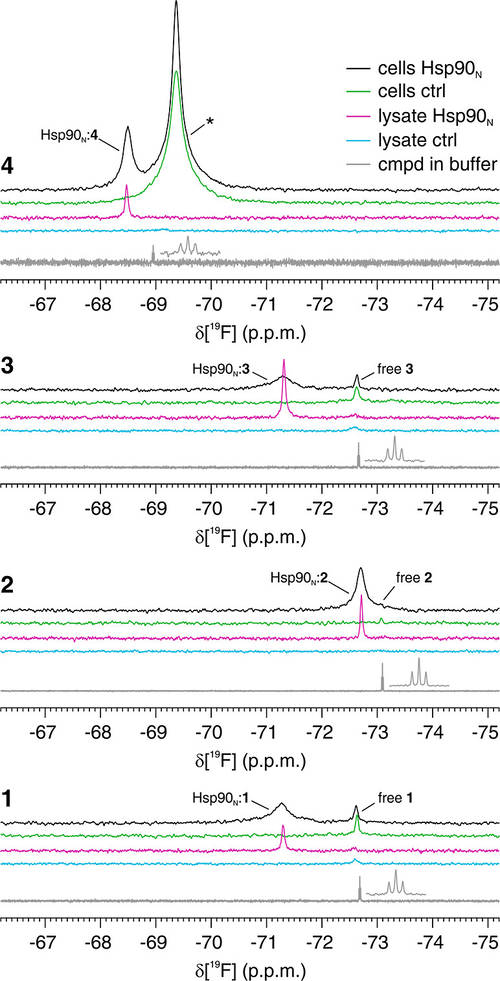 Figure 2. Fluorinated compounds bound to intracellular Hsp90N are detectable by19F NMR. In-cell (black, green) and lysate (magenta, cyan) 19F NMR spectra from cells expressing Hsp90N (black, magenta) and control cells (green, cyan) treated with compounds 1–4. Reference spectra of the pure compounds are shown in gray. The peaks attributed to the free and Hsp90N-bound compounds are labeled accordingly. An additional peak arising from the off-target binding of compound 4 is marked with an asterisk. (Luchinat et al., 2024)
Figure 2. Fluorinated compounds bound to intracellular Hsp90N are detectable by19F NMR. In-cell (black, green) and lysate (magenta, cyan) 19F NMR spectra from cells expressing Hsp90N (black, magenta) and control cells (green, cyan) treated with compounds 1–4. Reference spectra of the pure compounds are shown in gray. The peaks attributed to the free and Hsp90N-bound compounds are labeled accordingly. An additional peak arising from the off-target binding of compound 4 is marked with an asterisk. (Luchinat et al., 2024)
Case 2: Cosolvent dimethyl sulfoxide influences protein–ligand binding kinetics via solvent viscosity effects
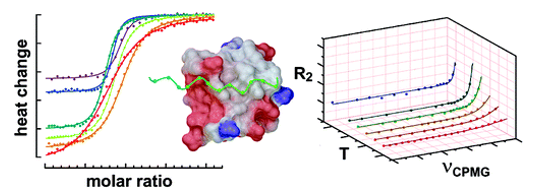
This study investigates how dimethyl sulfoxide (DMSO), commonly used to solubilize ligands, indirectly affects protein-ligand binding through changes in solvent viscosity. Using the carbohydrate recognition domain of galectin-3 as a model system, researchers examined binding to a drug-like ligand in varying concentrations of DMSO. Isothermal titration calorimetry revealed unchanged binding enthalpy but increasingly unfavorable entropy, leading to reduced binding affinity. 15N NMR relaxation showed that DMSO primarily slows the association rate of the ligand, while the dissociation rate remains largely unaffected. The association rate correlated inversely with viscosity and was significantly lower than the theoretical diffusion limit. The analysis suggests that only a small fraction of ligand-protein encounters result in successful binding, highlighting the influence of the solvent environment on binding kinetics and efficiency - important considerations for drug design.
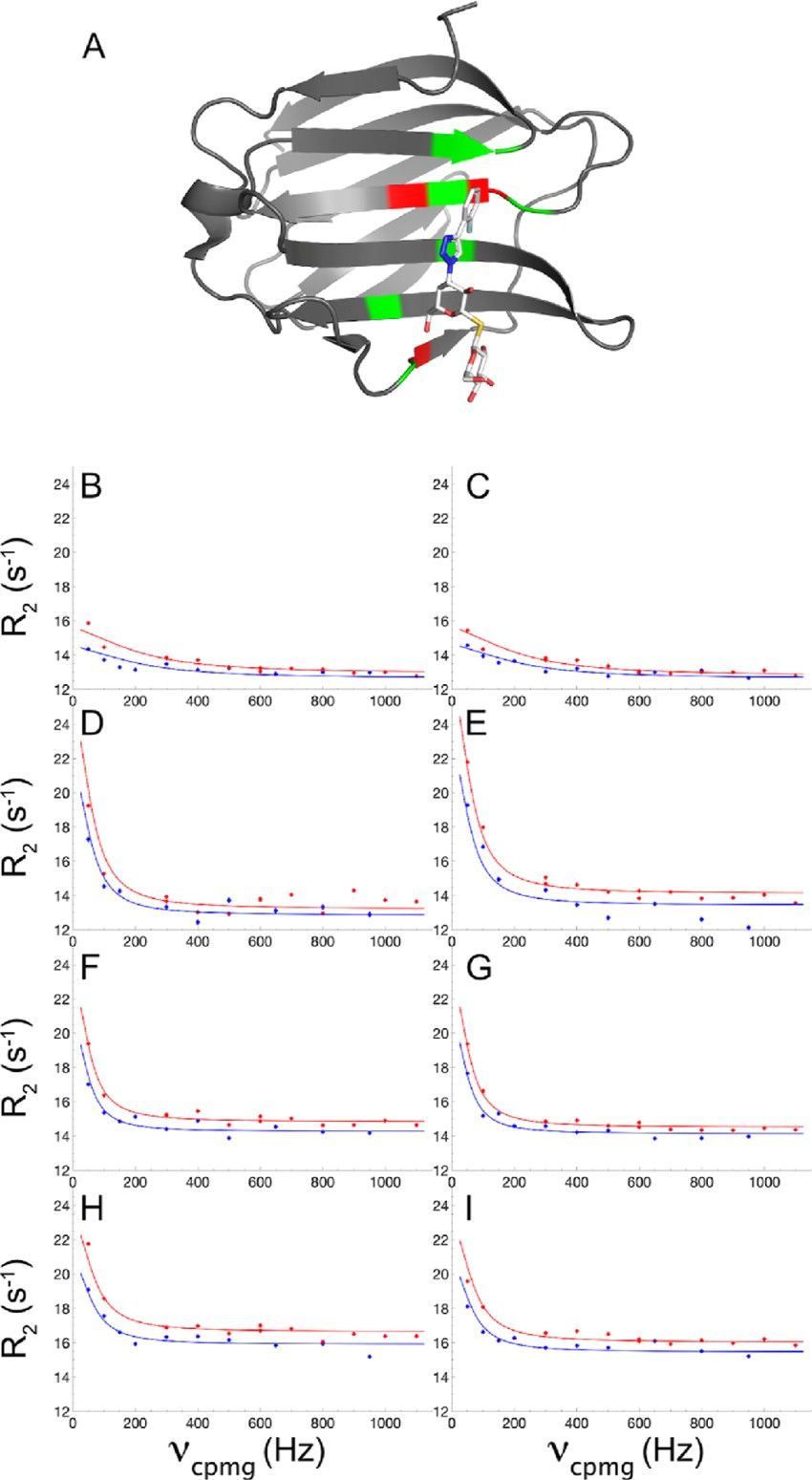 Figure 3. Ligand binding kinetics measured by NMR relaxation dispersion. (A) Crystal structure of the Gal3C−ligand complex with the protein and ligand shown in ribbon and stick representation, respectively. Protein residues showing significant relaxation dispersion at all the four DMSO concentrations are colored red: I145, L147, and E185. Protein residues with backbone atoms within 5 Å from the ligand are colored green. The ligand atoms are colored white (carbon), red (oxygen), blue (nitrogen), yellow (sulfur), and pale blue (fluorine). (B−I): 15 N CPMG relaxation dispersion profiles of residues I145 (B, D, F, H) and E185 (C, E, G, I) measured in 0% DMSO (B, C), 2% DMSO (D, E), 6% DMSO (F, G), and 10% DMSO (H, I). CPMG relaxation dispersions were acquired at static magnetic field strengths of 11.7 T (blue) and 14.1 T (red). Panel A was prepared using PDB-ID: 6RZF 46 and the Pymol software package. (Wernersson et al., 2023)
Figure 3. Ligand binding kinetics measured by NMR relaxation dispersion. (A) Crystal structure of the Gal3C−ligand complex with the protein and ligand shown in ribbon and stick representation, respectively. Protein residues showing significant relaxation dispersion at all the four DMSO concentrations are colored red: I145, L147, and E185. Protein residues with backbone atoms within 5 Å from the ligand are colored green. The ligand atoms are colored white (carbon), red (oxygen), blue (nitrogen), yellow (sulfur), and pale blue (fluorine). (B−I): 15 N CPMG relaxation dispersion profiles of residues I145 (B, D, F, H) and E185 (C, E, G, I) measured in 0% DMSO (B, C), 2% DMSO (D, E), 6% DMSO (F, G), and 10% DMSO (H, I). CPMG relaxation dispersions were acquired at static magnetic field strengths of 11.7 T (blue) and 14.1 T (red). Panel A was prepared using PDB-ID: 6RZF 46 and the Pymol software package. (Wernersson et al., 2023)
NMR spectroscopy provides a powerful set of tools for studying protein-ligand interactions at atomic resolution in solution. From detecting binding events and mapping interfaces to quantifying affinity and elucidating conformational dynamics, NMR remains indispensable in both basic research and translational applications such as drug discovery. While it requires careful sample preparation and expert interpretation, its ability to provide dynamic, kinetic, and structural insights makes it a uniquely versatile technique in the structural biology toolkit.
At Creative Biostructure, our specialized NMR services provide detailed insights into binding sites, affinities, and conformational changes critical to drug discovery, structural biology, and functional analysis. Let us help you unravel molecular interactions at atomic resolution. Contact us today to explore how our NMR expertise can advance your research and accelerate your innovation.
References
- Luchinat E, Barbieri L, Davis B, Brough PA, Pennestri M, Banci L. Ligand-based competition binding by real-time 19F NMR in human cells. J Med Chem. 2024;67(2):1115-1126. doi:10.1021/acs.jmedchem.3c01600
- Wernersson S, Birgersson S, Akke M. Cosolvent dimethyl sulfoxide influences protein–ligand binding kinetics via solvent viscosity effects: revealing the success rate of complex formation following diffusive protein–ligand encounter. Biochemistry. 2023;62(1):44-52. doi:10.1021/acs.biochem.2c00507
- Wu H, Paul F, Wehmeyer C, Noé F. Multiensemble Markov models of molecular thermodynamics and kinetics. Proc Natl Acad Sci USA. 2016;113(23). doi:10.1073/pnas.1525092113