What is Magnetic Circular Dichroism?
Magnetic Circular Dichroism (MCD) is a specialized spectroscopic technique that measures the difference in absorption between left and right circularly polarized light under an applied magnetic field. Unlike standard circular dichroism, which requires molecular chirality, MCD enables the study of both chiral and achiral systems by inducing magnetic optical activity. This method reveals detailed information about electronic transitions, spin configurations, orbital interactions, and molecular symmetry that conventional spectroscopy cannot detect.
How Magnetic Circular Dichroism Works
Differential Absorption of Circularly Polarized Light
At the core of MCD is the measurement of how a material absorbs left and right circularly polarized light differently under a magnetic field. This interaction induces electronic transitions, with the magnetic field altering the probabilities of absorption for each polarization. The resulting difference forms the MCD signal.
This effect is driven by Zeeman splitting, where degenerate energy levels split under a magnetic field. Even achiral materials exhibit induced optical activity, allowing MCD to reveal detailed electronic and magnetic properties otherwise inaccessible through conventional spectroscopy.
The Role of the Magnetic Field in MCD
The applied magnetic field is aligned parallel to the light propagation direction in most MCD experiments. This field perturbs the energy levels and modifies the selection rules that govern electronic transitions. It can induce optical activity in both chiral and achiral systems, making MCD uniquely versatile.
Beyond energy level splitting, the magnetic field also causes state mixing through spin-orbit and orbital angular momentum interactions. These effects give rise to asymmetric absorption that forms the distinctive MCD signal. Unlike linear absorption spectroscopy, MCD can detect both allowed and forbidden transitions, including weak or low-intensity signals that are otherwise difficult to observe.
Understanding the A, B, and C Terms in MCD
The MCD spectrum is composed of three theoretical contributions, each offering unique insights into the electronic and magnetic structure of the sample. These are known as the A-term, B-term, and C-term.
- A-terms (Diamagnetic Term): These terms arise from the Zeeman splitting of degenerate ground or excited states. They are typically dispersion-shaped (bipolar) and are independent of temperature. A-terms are characteristic of highly symmetric chromophores with degenerate electronic states, offering clues about the degeneracy and symmetry of the electronic transitions.
- B-terms (Paramagnetic Temperature-Independent Term): B-terms originate from the magnetic field-induced mixing of different electronic states (both ground and excited states). They are typically Gaussian or absorbance-like in shape and, like A-terms, are temperature-independent. B-terms are present in all molecules and provide information about the orbital angular momentum contributions and spin-orbit coupling within the electronic states.
- C-terms (Paramagnetic Temperature-Dependent Term): C-terms are arguably the most informative, particularly for paramagnetic systems. They arise from a population difference in the Zeeman-split components of a paramagnetic ground state. Because the population difference is dictated by the Boltzmann distribution, C-terms exhibit a strong temperature dependence, becoming more intense at lower temperatures. This temperature dependence is crucial for identifying paramagnetic species, determining their spin state, and even quantifying the magnetic moment of the ground state. A C-term signal is often absorbance-like in shape.
Theoretical and Computational Framework
The theoretical description of MCD is grounded in quantum mechanics, particularly perturbation theory, which models how a magnetic field alters the electronic structure of molecules. This framework provides the mathematical basis for understanding A-, B-, and C-term contributions.
To support experimental results, quantum chemical methods such as time-dependent density functional theory (TD-DFT) are often employed. These simulations help assign spectral features to specific transitions and predict MCD responses for new or hypothetical systems.
Computational analysis is especially useful for complex systems with overlapping spectral bands, helping researchers isolate meaningful contributions and correlate them with molecular structure and behavior.
Instrumentation and Spectral Regions in MCD
Magnetic Circular Dichroism experiments rely on precise instrumentation designed to detect subtle differences in light absorption under magnetic fields. A standard MCD setup includes the following core components:
Key Components of an MCD Spectrometer
- Broadband Light Source: Typically a xenon lamp or laser, selected based on the desired spectral range.
- Polarizers: Used to define the polarization state of the incoming light.
- Modulator: A photoelastic or electro-optic modulator alternates between left and right circularly polarized light.
- Sample Holder with Magnetic Control: Positioned within a variable magnetic field, usually generated by a superconducting magnet.
- Detector: A sensitive photodetector captures the differential absorption signals with high precision.
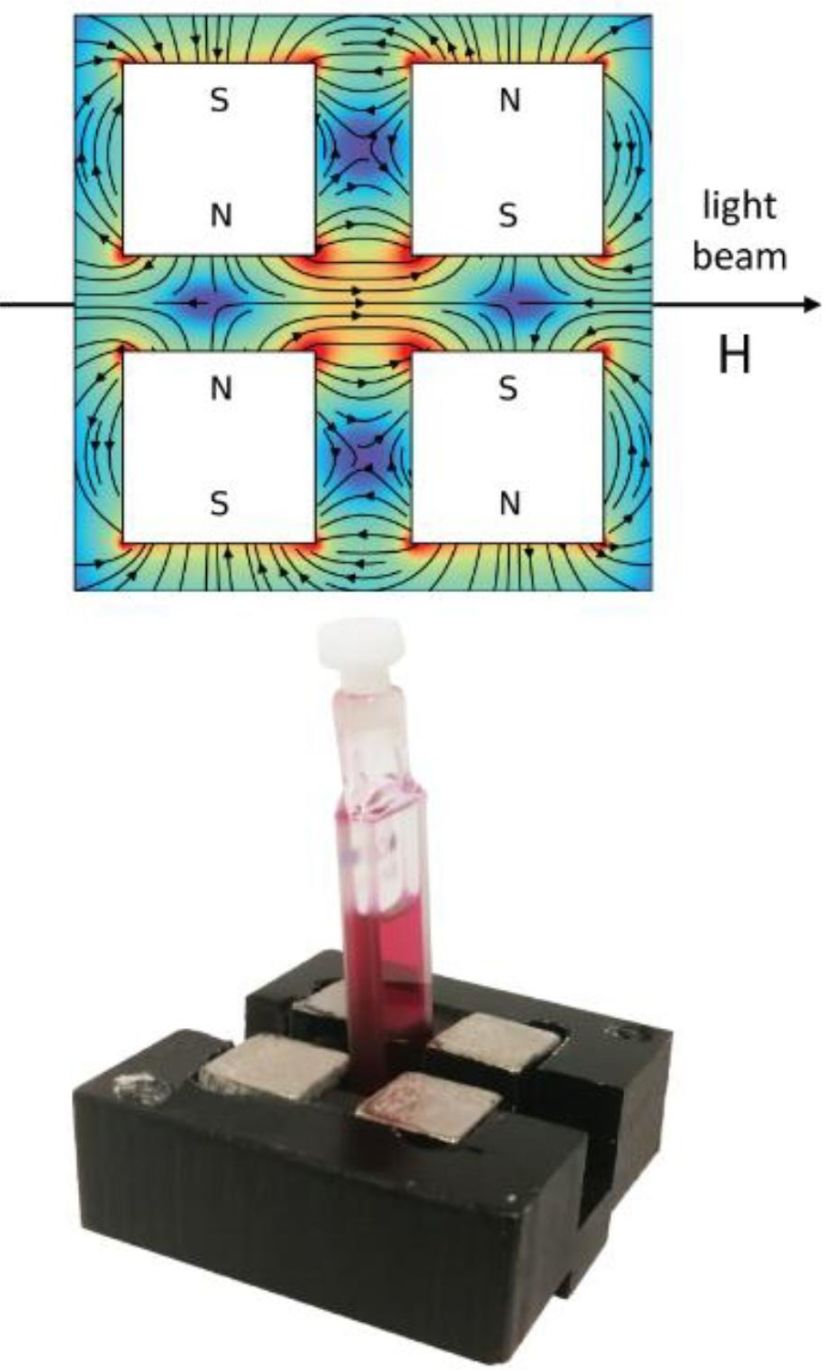 Figure 1. Cells and Holders Used for Magnetic Circular Dichroism (MCD) Measurements. (Fusè M, et al., 2024)
Figure 1. Cells and Holders Used for Magnetic Circular Dichroism (MCD) Measurements. (Fusè M, et al., 2024)
Spectral Coverage and Environmental Control
MCD measurements are typically performed across the ultraviolet (UV), visible (Vis), and near-infrared (NIR) regions. The optimal wavelength range depends on the material being studied and the specific electronic transitions of interest.
To ensure accurate and reproducible measurements, environmental parameters are tightly controlled:
- Magnetic Fields: Provided by superconducting magnets, often reaching several tesla in strength.
- Temperature Control: Cryostats are used to regulate temperature, which is critical for resolving C-term contributions in paramagnetic systems.
Advanced Magnetic Circular Dichroism Techniques
While conventional MCD provides valuable insight into electronic and magnetic properties, several advanced variants have emerged. These specialized techniques offer greater sensitivity, element specificity, or surface-focused measurements, making them essential for cutting-edge materials science and condensed matter research.
X-ray Magnetic Circular Dichroism Enhances Element Specificity
X-ray Magnetic Circular Dichroism (XMCD) is an extension of MCD into the X-ray region. It measures the difference in absorption between left and right circularly polarized X-rays at element-specific core-level absorption edges. This technique is uniquely capable of isolating the magnetic properties of individual elements within complex systems.
Key Features of XMCD
- Elemental specificity is achieved by tuning X-ray energies to target inner-shell transitions (e.g., 2p → 3d).
- Spin and orbital magnetic moments can be independently quantified using XMCD sum rules.
- Synchrotron radiation provides the high-intensity, circularly polarized X-rays required for these studies.
XMCD is particularly valuable in the analysis of 3d, 4f, and 5d elements, and is widely used in characterizing magnetic thin films, spintronic materials, and rare-earth compounds at the atomic level.
Reflective Magnetic Circular Dichroism Enables Surface Analysis
Reflective Magnetic Circular Dichroism (RMCD) is designed for samples that are opaque or exist as thin films on reflective substrates. Unlike transmission-based MCD, RMCD detects the differential reflection of circularly polarized light from a magnetized surface.
Applications of RMCD
- Ideal for surface-sensitive investigations of magnetic multilayers and interfaces
- Commonly used to analyze topological insulators, thin films, and nanostructures
- Provides access to electronic and magnetic surface states without requiring sample transparency
RMCD setups closely resemble traditional MCD systems but are configured for reflection geometry. This makes RMCD a preferred tool in nanotechnology and surface science where non-invasive probing is essential.
MCD Applications in Quantum Dots and Nanomaterials
Quantum dots (QDs) are semiconductor nanocrystals with discrete energy levels due to quantum confinement, making them ideal candidates for MCD studies. MCD spectroscopy reveals detailed information about spin states, excitonic transitions, and magneto-optical responses.
Insights from MCD in Quantum Dots
- Size-dependent magnetic behavior observed in materials like CdSe/ZnS and AgInS₂/ZnS
- Tunable optical and magnetic properties through control of QD size and composition
- Crucial for optoelectronic applications, including quantum computing and photonics
MCD thus functions as both a diagnostic and design tool in the development of next-generation nanomaterials.
Probing Quantum Geometry Using MCD
Quantum geometry, including Berry curvature and the quantum metric, underpins many phenomena in topological and correlated materials. Recent advancements have shown that MCD, combined with optical conductivity measurements, can reveal these complex geometrical features.
MCD and Quantum Geometry
- MCD enables mapping of Berry curvature effects in materials such as MnBi₂Te₄
- Helps to characterize topological phase transitions and electronic band topology
- Supports exploration of quantum anomalies in Weyl semimetals and other exotic systems
This frontier application of MCD is transforming how researchers understand quantum matter, enabling the design of materials with tailored topological and electronic properties.
What Is the Difference Between CD and MCD?
Circular Dichroism (CD) and Magnetic Circular Dichroism MCD both measure how circularly polarized light interacts with matter. However, they differ in core principles, sample requirements, and the types of molecular insights they offer. Understanding these differences helps researchers choose the right technique for structural or electronic analysis.
Chirality Versus Magnetically Induced Optical Activity
One of the most fundamental distinctions between CD and MCD lies in the role of chirality and magnetism.
Circular Dichroism Targets Intrinsic Chirality
CD spectroscopy is sensitive only to molecules with inherent chirality. This includes biomolecules like proteins and nucleic acids, whose asymmetry leads to different absorption of left and right circularly polarized light. CD spectroscopy is particularly effective for:
- Determining protein secondary structure (alpha-helices, beta-sheets)
- Monitoring conformational changes in biological macromolecules
- Assessing stereochemistry of chiral organic molecules
Select Service
- Secondary Structure Analysis
- Application of Circular Dichroism Spectroscopy in Protein Research
- Application of Circular Dichroism Spectroscopy in Nucleic Acid Research
- Application of Circular Dichroism Spectroscopy in Carbohydrate Research
- Application of Circular Dichroism Spectroscopy in Small Molecule Research
Related Reading
Magnetic Circular Dichroism Works Without Chirality
MCD, on the other hand, does not require the sample to be chiral. The application of an external magnetic field induces optical activity in any molecule, including symmetric and achiral systems. This enables MCD to probe a much wider range of materials, including:
- Metal-containing complexes and coordination compounds
- Paramagnetic centers in proteins and catalysts
- Inorganic and solid-state materials with magnetic properties
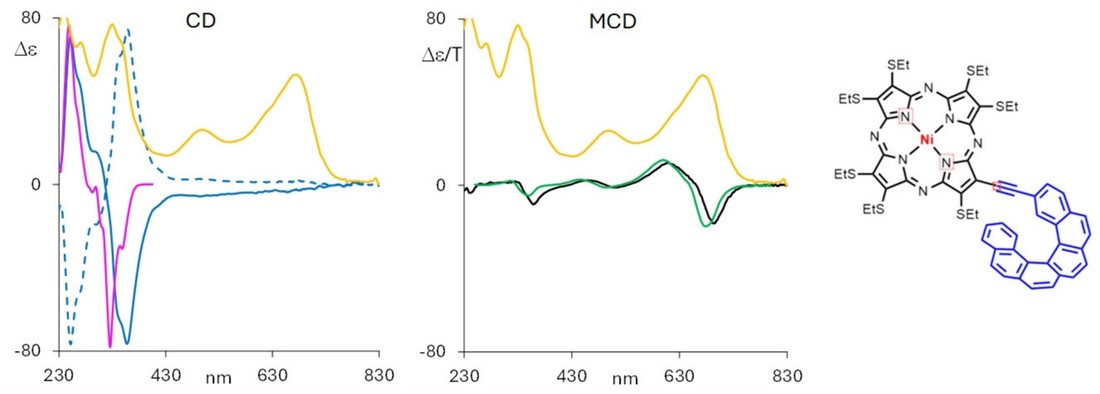 Figure 2. Natural and Magnetic CD Spectra of NiPzHelix Enantiomers. Left: Natural circular dichroism (CD) spectra of two enantiomers of helicene-substituted NiPzHelix. The solid blue line represents the (P) enantiomer; the dashed line represents its mirror image. The yellow line is the UV-Vis absorption spectrum, and the pink line shows the CD of the P-form carbo-hexahelicene. Right: Magnetic circular dichroism (MCD) spectrum of the M-form NiPzHelix (black) recorded under a 0.6 T magnetic field, compared with the MCD spectrum of a symmetric thioethyl porphyrazine Ni(II) complex (green). (Fusè M, et al., 2024)
Figure 2. Natural and Magnetic CD Spectra of NiPzHelix Enantiomers. Left: Natural circular dichroism (CD) spectra of two enantiomers of helicene-substituted NiPzHelix. The solid blue line represents the (P) enantiomer; the dashed line represents its mirror image. The yellow line is the UV-Vis absorption spectrum, and the pink line shows the CD of the P-form carbo-hexahelicene. Right: Magnetic circular dichroism (MCD) spectrum of the M-form NiPzHelix (black) recorded under a 0.6 T magnetic field, compared with the MCD spectrum of a symmetric thioethyl porphyrazine Ni(II) complex (green). (Fusè M, et al., 2024)
Information Revealed by CD and MCD
While both techniques provide structural insights, the depth and nature of the information differ considerably.
What CD Reveals
- Overall folding and secondary structure in biomolecules
- Chirality and conformational integrity
- Environmental changes affecting chromophores in solution
What MCD Reveals
- Electronic structure and energy level splitting in magnetic fields
- Oxidation states and spin states of transition metal centers
- Ligand field strength and coordination geometry
- Presence and behavior of paramagnetic species
- Orbital and spin angular momentum contributions
- Degeneracy and symmetry of electronic transitions
These distinctions make MCD especially valuable for studying metalloproteins, transition metal complexes, and advanced materials like quantum dots or spintronic compounds.
Experimental Setup and Measurement Considerations
The technical requirements for CD and MCD also vary.
CD Instrumentation
- Operates in the UV-Vis spectral range
- No magnetic field required
- Suitable for aqueous samples and biological systems
MCD Instrumentation
- Requires a high-strength magnetic field (often > 7 Tesla)
- Covers a broader spectral range including UV, visible, and near-infrared
- Incorporates cryogenic temperature control for C-term analysis in paramagnetic systems
Because of these setup differences, MCD is more demanding in terms of instrumentation but offers significantly richer spectroscopic detail, especially for electronically complex or magnetically active samples.
Summary of CD and MCD Differences
The following table summarizes the key differences in information content between CD and MCD:
| Aspect | Circular Dichroism (CD) | Magnetic Circular Dichroism (MCD) |
|---|---|---|
| Sensitivity to Chirality | Requires intrinsic chirality | Induces optical activity; works on both chiral and achiral systems |
| Primary Application | Structural biology, conformational analysis | Electronic structure, spin states, magnetic behavior |
| Information Provided | Protein folding, nucleic acid conformation, chirality | Oxidation/spin states, ligand field effects, orbital and spin angular momentum |
| Sample Types | Chiral biological molecules | Paramagnetic complexes, metal centers, quantum materials |
| Signal Dependence | Intrinsic molecular asymmetry | Zeeman splitting and magnetic field–induced transitions |
| Temperature Sensitivity | Usually measured at room temperature | Often performed at cryogenic temperatures (especially for C-term detection) |
Applications of Magnetic Circular Dichroism
Unraveling Biological Macromolecules
MCD is particularly powerful for studying biological systems containing metal ions, which are often at the heart of enzymatic activity and biological electron transfer.
- Metalloproteins and Heme Proteins: MCD is a cornerstone technique for characterizing the electronic and magnetic properties of metal centers in metalloproteins. For example, in heme proteins like cytochromes and hemoglobin, MCD can precisely determine the oxidation state and spin state of the iron center, identify axial ligands, and monitor conformational changes that occur during ligand binding or catalytic cycles. Its sensitivity to subtle electronic changes makes it invaluable for understanding enzyme active site mechanisms and the electronic structure of metal cofactors.
- Enzyme Active Site Elucidation: For enzymes containing transition metal ions (e.g., copper, iron, manganese), MCD can provide crucial information about the coordination environment and electronic structure of the catalytic site, helping to elucidate reaction intermediates and catalytic mechanisms.
- Conformational Changes: While CD is primary for protein secondary structure, MCD can sometimes detect conformational changes that alter the electronic environment of a chromophore, especially if a paramagnetic center is involved.
Pushing the Boundaries in Materials Science
MCD is a vital technique for characterizing the electronic and magnetic properties of novel materials, especially those with potential for spintronics, quantum computing, and advanced optical applications.
- Semiconductor Quantum Dots (MCD quantum dots): MCD is exquisitely sensitive to the discrete electronic transitions and exciton states within semiconductor quantum dots. It can provide information about their size, shape, surface passivation, and the influence of quantum confinement on their electronic structure and magnetic properties.
- Magnetic Materials: For a wide range of magnetic materials, including thin films, nanoparticles, and bulk samples, MCD (and especially XMCD) offers detailed insights into their magnetic anisotropy, magnetic ordering, and the contributions of spin and orbital moments to the overall magnetization. This is crucial for developing new magnetic storage devices and spintronic technologies.
- Novel Electronic Materials and Spintronics: Researchers use MCD to study the electronic structure of topological insulators, graphene, and other emerging materials, where the interplay between electronic and magnetic properties leads to novel phenomena.
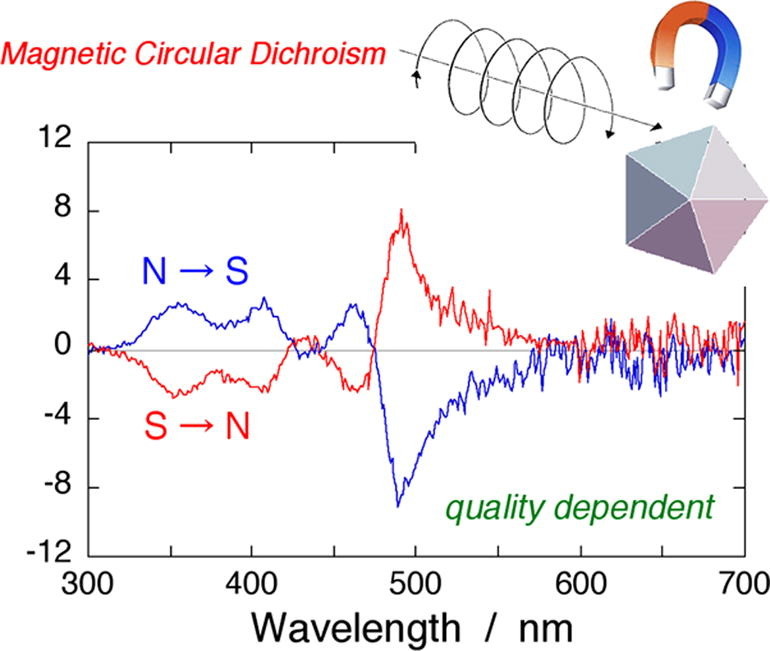 Figure 3. Morphological Quality Assessment of Silver Nanodecahedra via Magnetic Circular Dichroism. (Sato H, et al., 2019)
Figure 3. Morphological Quality Assessment of Silver Nanodecahedra via Magnetic Circular Dichroism. (Sato H, et al., 2019)
Inorganic Chemistry and Coordination Compounds
In the realm of synthetic inorganic chemistry, MCD is an indispensable tool for:
- Electronic Structure of Transition Metal and f-Element Complexes: It provides detailed information about the electronic energy levels, ligand field parameters, and spin-orbit coupling in a wide variety of transition metal and lanthanide complexes. This helps validate theoretical calculations and understand bonding characteristics.
- Ligand Field Theory and Structural Elucidation: By analyzing the A, B, and C terms, chemists can gain insights into the symmetry of the coordination environment around a metal ion, assisting in the elucidation of complex molecular structures.
Organic Molecules
While often associated with metal centers, MCD can also be applied to non-chiral organic chromophores that possess significant orbital angular momentum or undergo state mixing in a magnetic field. It can provide complementary information to absorption spectroscopy, helping to resolve overlapping electronic transitions and characterize excited-state properties.
A study demonstrates that MCD can provide valuable electronic structure insights even for non-chiral organic chromophores like nucleosides. Unlike Electronic Circular Dichroism (ECD), which depends on molecular chirality, MCD signals originate from intrinsic π–π* transitions in the aromatic bases (e.g., guanine, adenine). Experimental and simulated spectra show strong agreement, and MCD remains stable under molecular dynamics averaging, highlighting its robustness and suitability for probing electronic transitions in flexible or achiral systems.
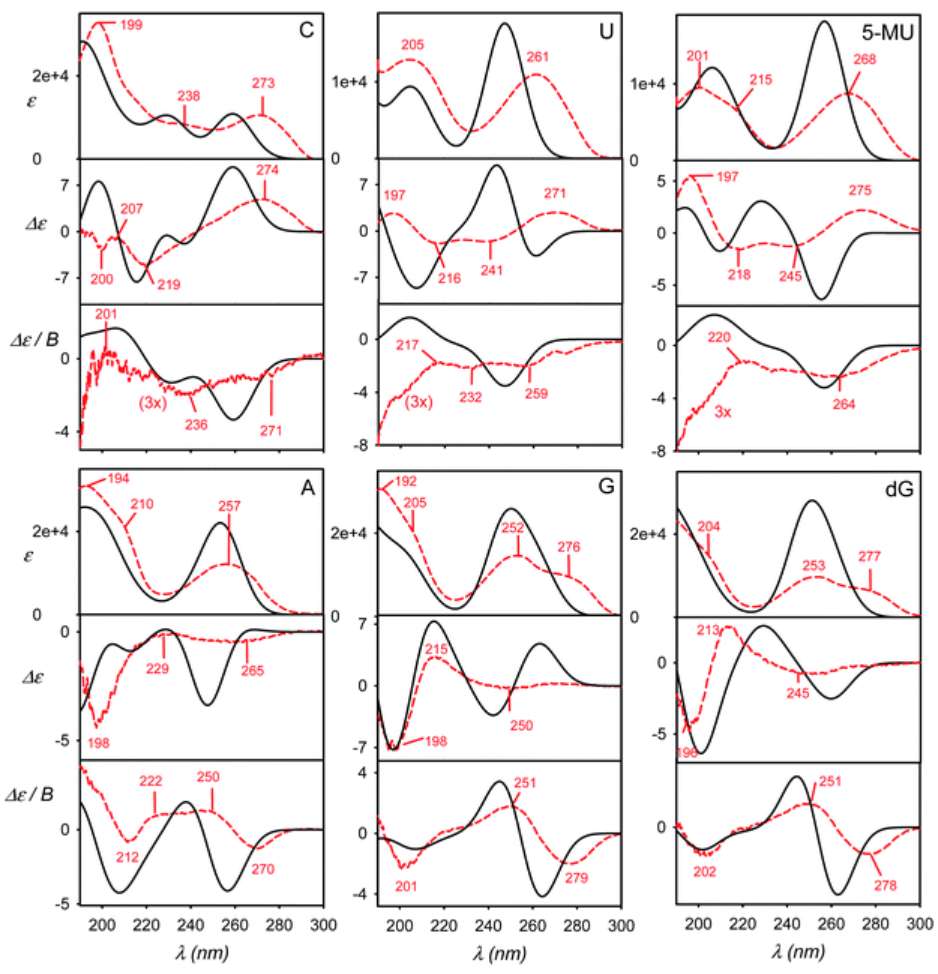 Figure 4. Calculated (black, solid) and experimental (red, dashed) absorption (ε), electronic circular dichroism (ECD, Δε), and magnetic circular dichroism (MCD, Δε/B) spectra of six nucleosides: cytidine (C), uridine (U), 5-methyluridine (5-MU), adenosine (A), guanosine (G), and deoxyguanosine (dG). (Kaminský J, et al., 2021)
Figure 4. Calculated (black, solid) and experimental (red, dashed) absorption (ε), electronic circular dichroism (ECD, Δε), and magnetic circular dichroism (MCD, Δε/B) spectra of six nucleosides: cytidine (C), uridine (U), 5-methyluridine (5-MU), adenosine (A), guanosine (G), and deoxyguanosine (dG). (Kaminský J, et al., 2021)
In summary, MCD is a versatile and sophisticated technique that bridges spectroscopy, magnetism, and quantum theory. Its sensitivity to electronic structure, spin interactions, and quantum geometry makes it indispensable in modern research. With ongoing advancements in instrumentation and computational analysis, MCD is poised to play an increasingly vital role in the exploration of functional materials and quantum systems.
Whether you're studying metalloproteins, quantum dots, or novel magnetic materials, MCD delivers unparalleled insights that complement traditional spectroscopic tools. At Creative Biostructure, we provide professional MCD and CD spectroscopy services with advanced instrumentation and expert analysis. Contact us to discuss your project needs and discover how our tailored solutions can accelerate your research.
References
- Vickery L, Nozawa T, Sauer K. Magnetic circular dichroism studies of myoglobin complexes. Correlations with heme spin state and axial ligation. Journal of the American Chemical Society. 1976, 98(2): 343-350.
- Martin S R, Schilstra M J. Circular dichroism and its application to the study of biomolecules. Methods in Cell Biology. 2008, 84: 263-293.
- Sato H, Yao H. Application of magnetic circular dichroism (MCD) to morphological quality evaluation of silver nanodecahedra. Chemical Physics Letters. 2019, 732: 136637.
- Kaminský J, Andrushchenko V, Bouř P. Natural and magnetic circular dichroism spectra of nucleosides: effect of the dynamics and environment. RSC Advances. 2021, 11(14): 8411-8419.
- Sumida K, Takeda Y, Kusaka S, et al. Short-range magnetic interaction in a monolayer 1 T-VSe 2 film revealed by element-specific x-ray magnetic circular dichroism. Physical Review Materials. 2022, 6(1): 014006.
- Fusè M, Mazzeo G, Ghidinelli S, et al. Experimental and theoretical aspects of magnetic circular dichroism and magnetic circularly polarized luminescence in the UV, visible and IR ranges: A review. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2024: 124583.
- Sun R, Feng B, Gao X. Magnetic Circular Dichroism of Chiral Quantum Rods. The Journal of Physical Chemistry C. 2025, 129(14): 6835-6839.