Drug receptors—proteins, nucleic acids, or other biomolecules—mediate the pharmacological effects of therapeutics by binding to specific ligands. Understanding receptor behavior at atomic resolution is essential for elucidating mechanisms of action (MOA), resistance, and off-target effects. Nuclear Magnetic Resonance (NMR) spectroscopy is uniquely positioned to dissect receptor structure, dynamics, and interaction networks in solution or near-native environments.
Creative Biostructure offers comprehensive NMR spectroscopy services including for drug receptor analysis. Learn more about with us advanced NMR strategies for direct receptor analysis, emphasizing conformational changes, allostery, and binding thermodynamics, while avoiding overlap with ligand-centric screening techniques.
 Figure 1. Maraviroc binding to the HIV1- coreceptor, CC chemokine receptor 5 (CCR5), prevents it from binding the viral envelope protein gp120, blocking a HIV infection. PDB code: 4mbs.
Figure 1. Maraviroc binding to the HIV1- coreceptor, CC chemokine receptor 5 (CCR5), prevents it from binding the viral envelope protein gp120, blocking a HIV infection. PDB code: 4mbs.
NMR Principles Relevant to Receptor Studies
NMR detects signals from nuclei (e.g., 1H, 13C, 15N) in a magnetic field. Key concepts:
- Chemical Shift: Reflects local electronic environment, used to map binding interfaces and conformational states.
- Dipolar Coupling & NOE: Provides distance restraints (<5 Å) for 3D structure determination.
- Relaxation Rates (T1/T2): Report on receptor flexibility and timescale dynamics (ps to ms).
- Paramagnetic Probes: Spin labels or metal ions enable long-distance measurements (PRE, pseudocontact shifts).
For receptors, isotopic labeling (15N, 13C) and advanced pulse sequences (e.g., TROSY, CRINEPT) are critical to resolve large, flexible systems.
Key Applications in Receptor Analysis
A comprehensive understanding of receptors—key cellular mediators involved in processes ranging from neurotransmission to immune regulation—requires analytical techniques capable of capturing both structural and dynamic information. NMR spectroscopy offers a distinct advantage in this regard, enabling the observation of receptor behavior in conditions that closely mimic, or even replicate, the native cellular environment. Explore the critical role of NMR in advancing receptor research.
Receptor Structure Determination
- Intrinsically Disordered Receptors (IDRs): Many receptors, like the N-terminal domains of G protein-coupled receptors (GPCRs), refuse to adopt a single rigid shape. NMR techniques such as 1H-15N HSQC (Heteronuclear Single Quantum Coherence) and relaxation experiments allow researchers to probe the fleeting, flexible conformations that these regions explore in solution. These insights are critical for understanding how such "unstructured" regions regulate signaling and ligand binding.
- Multidomain Proteins: NMR spectroscopy utilizes residual dipolar couplings (RDCs), measured in weakly aligned media, to determine the relative orientations and motions of individual domains within multidomain proteins. This approach provides critical insights into the dynamic interplay between domains, offering a more comprehensive understanding of protein conformational flexibility beyond static structural snapshots.
- Membrane-Embedded Receptors: Membrane proteins present significant challenges for traditional structural biology methods due to their intrinsic hydrophobicity and conformational heterogeneity. Solid-state NMR, performed in native-like environments such as lipid bilayers or nanodiscs, enables high-resolution structural and dynamic analysis of membrane-bound receptors—including the β2-adrenergic receptor—without requiring crystallization. This technique allows for the investigation of receptors in physiologically relevant states, including ligand-bound conformations.
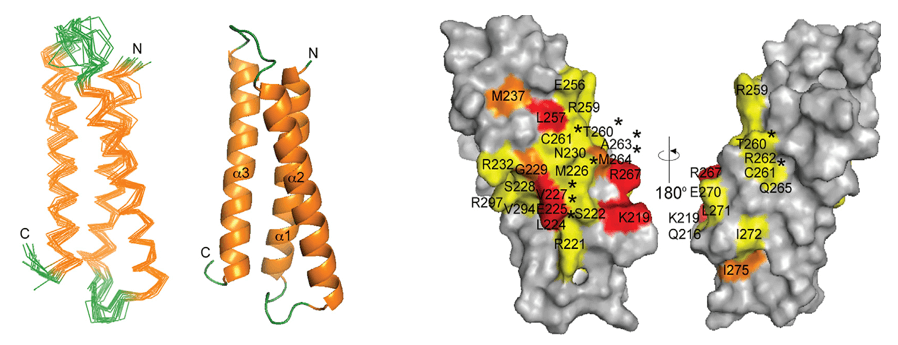
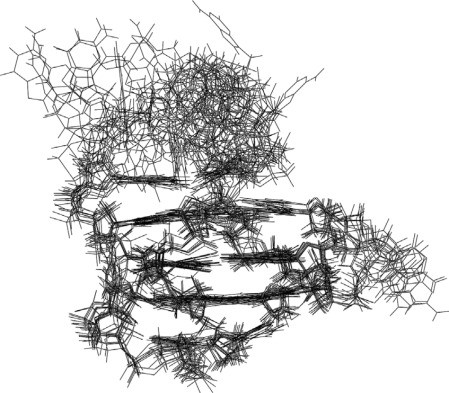 Figure 2. An ensemble of 10 structures from an NMR determination, of a DNA quadruplex formed by a sequence found in the human bcl2 promoter (PDB entry no. 2F8U). Note the close correspondence of the bases in the various structures, contrasting with the diversity of conformation and flexibility shown by the extrahelical groups. (Neidle, 2008)
Figure 2. An ensemble of 10 structures from an NMR determination, of a DNA quadruplex formed by a sequence found in the human bcl2 promoter (PDB entry no. 2F8U). Note the close correspondence of the bases in the various structures, contrasting with the diversity of conformation and flexibility shown by the extrahelical groups. (Neidle, 2008)
Related Reading
- MemPro™ Membrane Protein Families
- Custom MemPro™ GPCR Structure-Function Services
- Custom MemPro™ Ion Channel Services
- MemPro™ Rhodopsin-like Receptors Services
- Custom MemPro™ Pentameric Ligand-gated Ion Channels Services
- Custom MemPro™ Services for Tumor necrosis factor receptor
- MemPro™ VEGF receptors
- MemPro™ Chemokine Receptor 8 Protein
- Structural Research of Novel Receptors
Binding Thermodynamics and Kinetics
- Affinity (Kd): NMR spectroscopy enables the quantification of binding affinities through chemical shift perturbation (CSP) analysis during ligand titration experiments. By monitoring and plotting the changes in resonance frequencies of receptor nuclei upon ligand binding, dissociation constants (Kd) can be determined with high accuracy and sensitivity.
- Kinetics (kon/koff): Binding kinetics, including association (kon) and dissociation (koff) rates, can be elucidated using NMR techniques such as exchange saturation transfer (EST) and ZZ-exchange experiments. These methods provide real-time insights into the dynamic nature of molecular interactions, which are particularly valuable in the context of drug discovery and optimization.
- Entropy/Enthalpy Dissection: A comprehensive understanding of binding thermodynamics can be achieved by integrating NMR with isothermal titration calorimetry (ITC). This combination allows for the decomposition of free energy changes into enthalpic (ΔH) and entropic (ΔS) contributions, offering mechanistic insights into the driving forces underlying molecular recognition and complex formation.
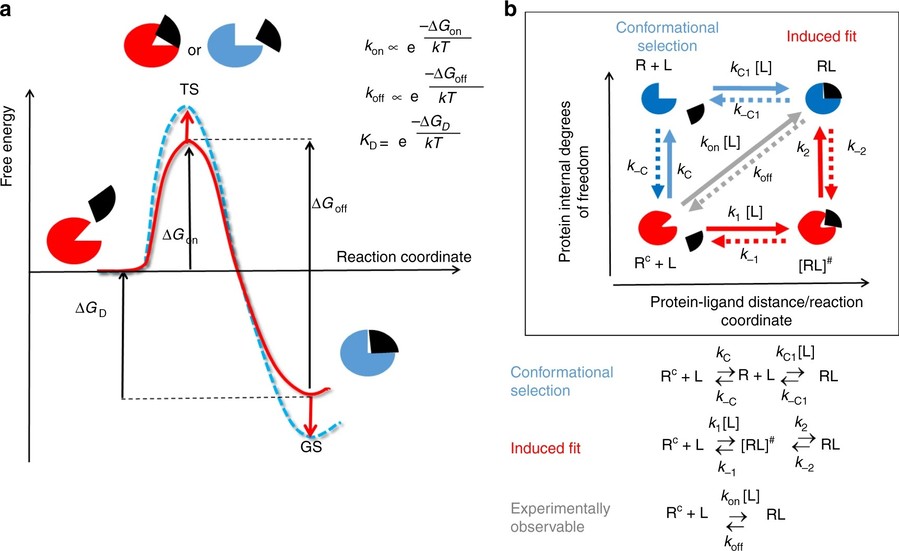 Figure 3. Models of drug–target binding. a Schematic diagram of a one-barrier drug–target binding free energy profile. b Diagram schematically illustrating different mechanisms of drug binding involving protein conformational changes. (Amaral et al., 2017)
Figure 3. Models of drug–target binding. a Schematic diagram of a one-barrier drug–target binding free energy profile. b Diagram schematically illustrating different mechanisms of drug binding involving protein conformational changes. (Amaral et al., 2017)
Conformational Dynamics and Allostery
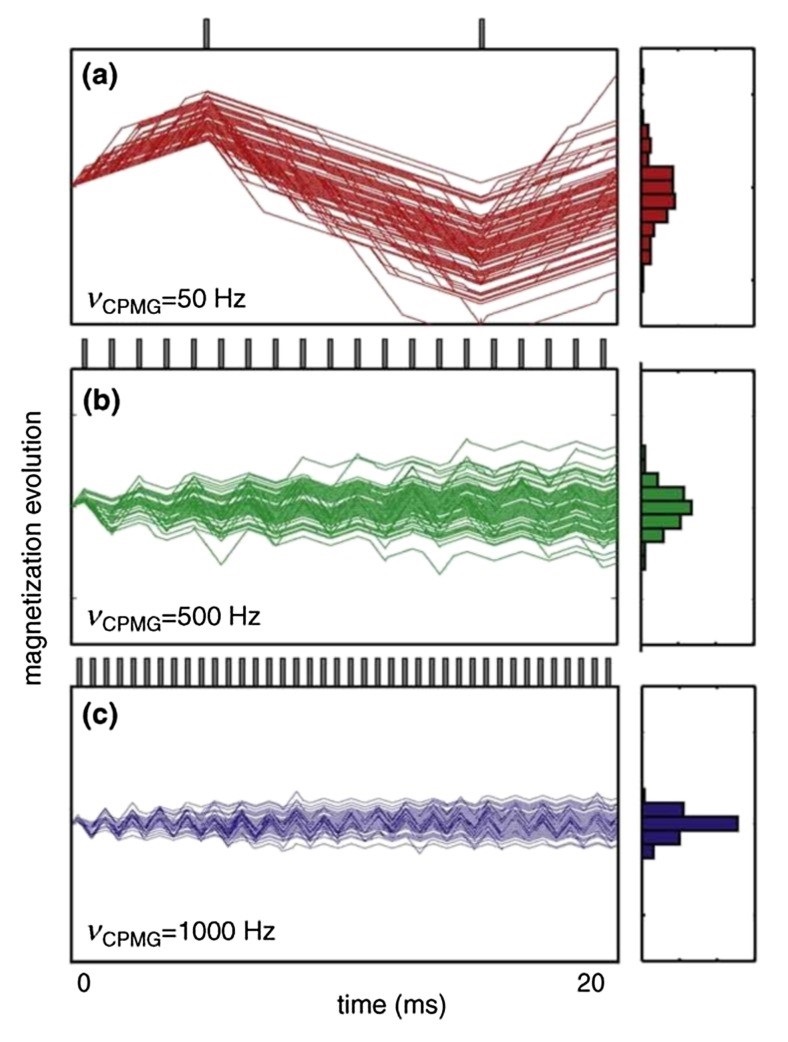
- Slow Motions (μs-ms): Functionally critical conformational changes in proteins, such as allosteric transitions, often occur on the microsecond to millisecond timescale. NMR relaxation dispersion experiments are uniquely suited to detect and characterize these low-populated, transient conformational states, providing insight into the dynamic equilibria that govern receptor activation and regulation.
- Fast Motions (ps-ns): Fast, localized protein dynamics occurring on the picosecond to nanosecond timescale can be investigated using model-free analysis of 15N relaxation data. These motions, including loop flexibility and hinge bending, play essential roles in modulating receptor flexibility, molecular recognition, and overall functional responsiveness.
- Allosteric Pathways: Allosteric regulation involves long-range communication within a protein structure, where conformational changes in one region influence distant functional sites. Techniques such as methyl-TROSY and chemical shift perturbation (CSP) mapping enable the identification of allosteric pathways by tracking structural and dynamic changes across domains—for example, signal propagation between ATP-binding pockets and substrate recognition sites in kinases.
 Figure 4. NMR can ascertain the distribution of dynamics on the ligand to identify the part of ligand that need to optimize. (Dubey et al., 2020)
Figure 4. NMR can ascertain the distribution of dynamics on the ligand to identify the part of ligand that need to optimize. (Dubey et al., 2020)
Oligomerization and Aggregation
- Self-Association: Diffusion-ordered spectroscopy (DOSY) estimates molecular size by tracking how fast molecules move through a solution, revealing shifts in hydrodynamic radius upon oligomerization.
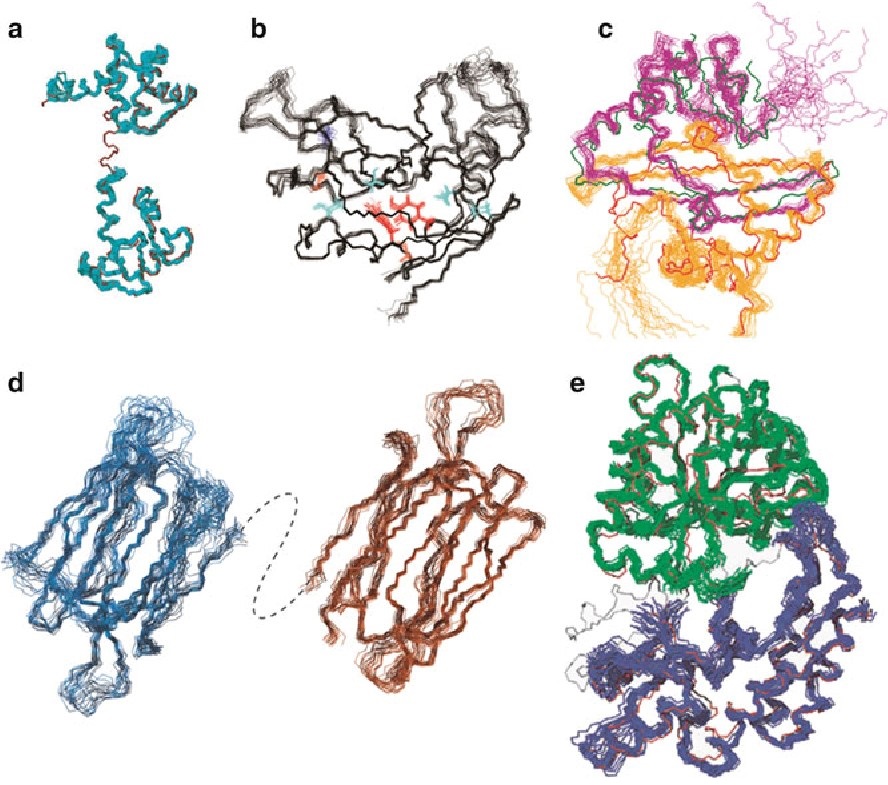
- Amyloid Formation: For receptors prone to misfolding or involved in neurodegenerative disease pathways, NMR can monitor β-sheet transitions associated with amyloid formation, using 13C-detected experiments. This is crucial in studying proteins like prions or tau.
 Figure 5. Solution NMR reveals amyloid formation. (Karamanos et al., 2015)
Figure 5. Solution NMR reveals amyloid formation. (Karamanos et al., 2015)
Post-Translational Modifications (PTMs)
Receptors are like modular machines, with fine-tuned control switches in the form of phosphorylation, acetylation, or ubiquitination.
- Using 31P NMR, scientists can directly observe phosphorylation events.
- Alternatively, CSP mapping tracks structural changes due to PTMs. A great example is EGFR, where phosphorylation of its activation loop flips the receptor from dormant to "go-time" mode.
 Figure 6. Characterizing post-translational modifications and their effects on protein conformation using NMR spectroscopy. (Kumar et al., 2020)
Figure 6. Characterizing post-translational modifications and their effects on protein conformation using NMR spectroscopy. (Kumar et al., 2020)
In-Cell NMR
Finally, receptors live in cells—not in buffers. In-cell NMR offers the rare privilege of watching them in their native habitat.
- Using 19F labels or hyperpolarized 13C probes, researchers can capture how receptors behave under the crowded, complex conditions inside living cells. This technique reveals real-time, native-state interactions, which are often missed in vitro.
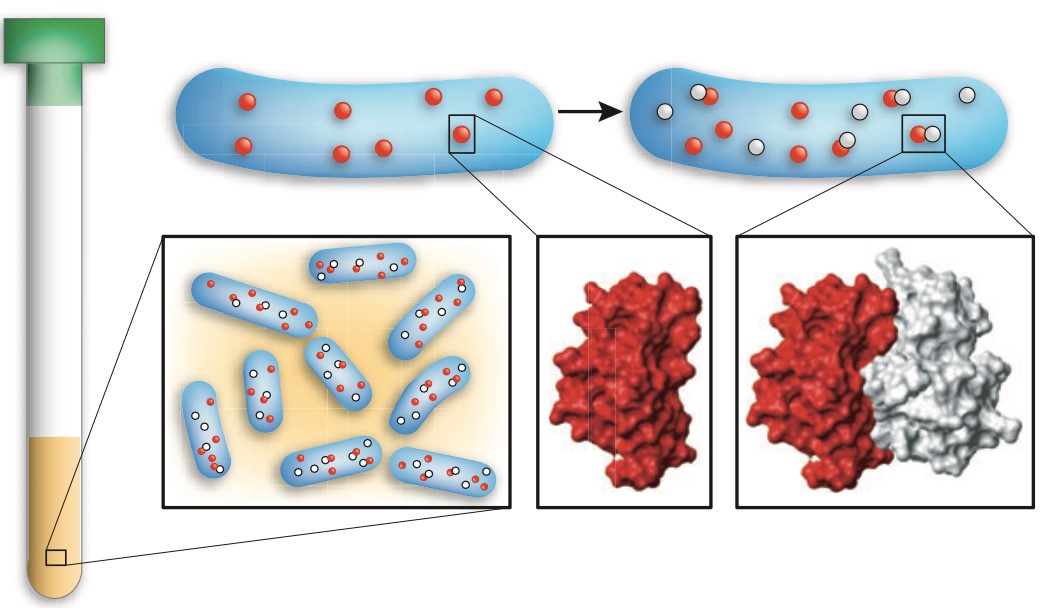
 Figure 7. In-cell NMR is applied to detect the conformational changes of cytosolic proteins in live cells. (A) The vector containing a gene of interest (green arrow) is delivered by transient transfection in labeled medium. Cells expressing the protein of interest (green) are collected and placed in a 3 mm Shigemi NMR tube and applied for in-cell NMR analysis. (B-D) 1H−15N NMR spectra of SOD1 in the apo and reduced state (B), the one-zinc-ion (cyan)-per-monomer bound and dimerized state (C) and the fully mature, copper (salmon)-zinc (cyan)-bound and disulfide-oxidized state (D) in human cells. Figure adapted from ref. with permission. (Zhang et al., 2021)
Figure 7. In-cell NMR is applied to detect the conformational changes of cytosolic proteins in live cells. (A) The vector containing a gene of interest (green arrow) is delivered by transient transfection in labeled medium. Cells expressing the protein of interest (green) are collected and placed in a 3 mm Shigemi NMR tube and applied for in-cell NMR analysis. (B-D) 1H−15N NMR spectra of SOD1 in the apo and reduced state (B), the one-zinc-ion (cyan)-per-monomer bound and dimerized state (C) and the fully mature, copper (salmon)-zinc (cyan)-bound and disulfide-oxidized state (D) in human cells. Figure adapted from ref. with permission. (Zhang et al., 2021)
Select Service
Advanced NMR Methodologies for Receptor Analysis
| Strategies | Methods | Description |
|---|---|---|
| Isotope Labeling Strategies | Segmental Labeling | Study specific domains in large receptors (e.g., TCR-CD3 complex). |
| Methyl-Specific Labeling | ILV (isoleucine, leucine, valine) 13CH3 probes enhance sensitivity for high-molecular-weight systems. | |
| Hyperpolarization | Dissolution DNP | Amplifies signal for low-abundance receptors (e.g., membrane proteins in nanodiscs). |
| Paramagnetic NMR | Cysteine-Specific Spin Labels | PRE measurements map ligand-binding pockets or domain arrangements. |
| Lanthanide Tags | Pseudocontact shifts orient helices or loops in multidomain receptors. | |
| Integrated Hybrid Approaches | NMR-Cryo-EM Fusion | Combines NMR dynamics with cryo-EM static structures (e.g., HIV envelope glycoprotein). |
| MD Simulations Guided by NMR Restraints | Refines conformational ensembles (e.g., Aβ42 fibrils). |
Case Studies
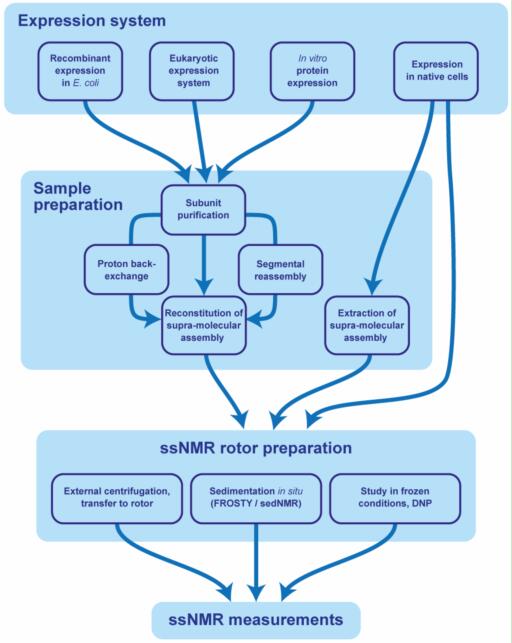
Case 1: G Protein-coupled receptor (GPCR) reconstitution and labeling for solution nuclear magnetic resonance (NMR) studies of the structural basis of transmembrane signaling
G protein-coupled receptors (GPCRs) are vital membrane proteins in higher organisms that regulate physiological responses to external signals, making them key drug targets. Their signaling depends on structural and dynamic changes influenced by ligands and intracellular partners like G proteins and arrestins. This review highlights how solution-state nuclear magnetic resonance (NMR) spectroscopy is used to study GPCR conformations and interactions relevant to their signaling functions.
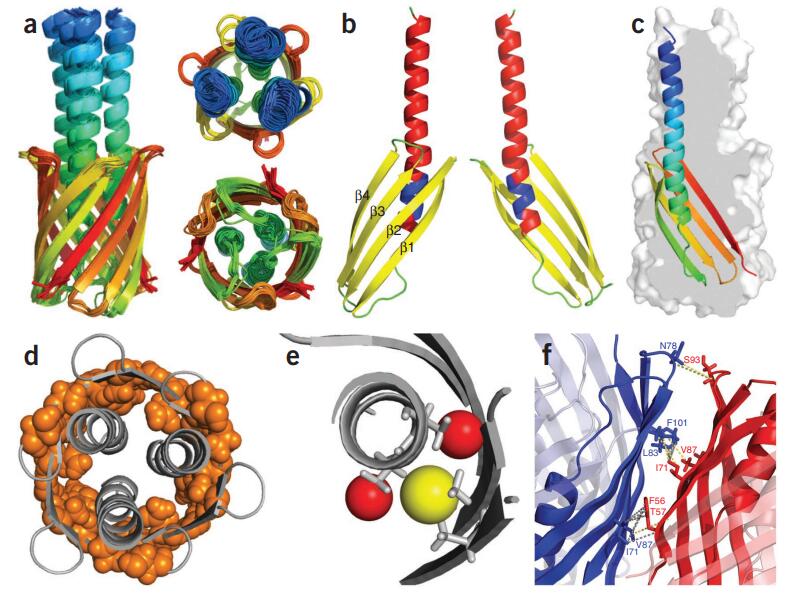
 Figure 8. Sequence-specific assignment of the 19F-NMR signals of TET moieties attached to the three native Cys residues near the intracellular surface of the transmembrane domain (TMD) of the GLP-1R. (A) Crystal structure of the GLP-1R[TMD] (PDB: 5VEX) shown as a green cartoon. Three native Cys residues, C174, C329 and C341, shown as black spheres, are exposed on the intracellular surface and are thus accessible for TET labeling with the IMCM method. (B) 1D 19F-NMR spectra. The GLP-1R[TMD] spectrum contains three signals corresponding to the three Cys sites shown in (A). Individual assignments of the 19F-NMR signals were obtained using site-specific mutagenesis to replace cysteines with serine residues. The peak assignments indicated at the top by the one-letter amino acid code and the residue number are based on the disappearance of the signals of the replaced cysteines. (Ge et al., 2022)
Figure 8. Sequence-specific assignment of the 19F-NMR signals of TET moieties attached to the three native Cys residues near the intracellular surface of the transmembrane domain (TMD) of the GLP-1R. (A) Crystal structure of the GLP-1R[TMD] (PDB: 5VEX) shown as a green cartoon. Three native Cys residues, C174, C329 and C341, shown as black spheres, are exposed on the intracellular surface and are thus accessible for TET labeling with the IMCM method. (B) 1D 19F-NMR spectra. The GLP-1R[TMD] spectrum contains three signals corresponding to the three Cys sites shown in (A). Individual assignments of the 19F-NMR signals were obtained using site-specific mutagenesis to replace cysteines with serine residues. The peak assignments indicated at the top by the one-letter amino acid code and the residue number are based on the disappearance of the signals of the replaced cysteines. (Ge et al., 2022)
Case 2: High-resolution magic angle spinning NMR of KcsA in liposomes: the highly mobile c-terminus
This study uses high-resolution magic angle spinning (HR-MAS) NMR and fractional deuteration to investigate the elusive cytosolic N- and C-terminal regions of the bacterial potassium channel KcsA embedded in lipid membranes. 1H-detected HR-MAS NMR reveals that the C-terminus becomes more dynamic under acidic conditions, supporting models where it stabilizes the channel tetramer at neutral pH. New residue assignments in the lipid-embedded C-terminus are reported, and a truncation mutation is shown to destabilize the selectivity filter. Additionally, the study notes lipid hydrolysis during routine experimental conditions, highlighting an important factor for interpreting in-membrane NMR data.
 Figure 9. 1H-15N HSQC by HR-MAS of full-length wild-type KcsA (red), and KcsA-∆125 (navy); both samples pH 7.25, 50 mM K+, 308 K, 5 kHz MAS. (Howarth and McDermott, 2022)
Figure 9. 1H-15N HSQC by HR-MAS of full-length wild-type KcsA (red), and KcsA-∆125 (navy); both samples pH 7.25, 50 mM K+, 308 K, 5 kHz MAS. (Howarth and McDermott, 2022)
Select Service
Challenges in Receptor-Focused NMR
- Sensitivity: Receptors, including GPCRs, receptor tyrosine kinases, and ion channels, are typically large (>50 kDa) and structurally flexible, presenting significant challenges for conventional solution-state NMR spectroscopy. As molecular weight increases, signal sensitivity and resolution decline due to enhanced transverse relaxation rates and spectral overlap, complicating data interpretation.
- Membrane Mimetics: Receptors such as GPCRs are naturally embedded within lipid bilayers, and replicating this native environment in vitro without compromising structural integrity or functional activity remains challenging. Various membrane-mimetic systems—including detergents, micelles, bicelles, liposomes, and nanodiscs—have been developed to approximate physiological conditions, yet each comes with inherent limitations, and no universally optimal model has emerged.
- Transient Interactions: Many receptor-associated interactions, particularly those involved in signal transduction pathways, are inherently transient and dynamic. Examples include short-lived GPCR–arrestin complexes or rapid conformational shifts induced by ligand binding. Capturing these fleeting events requires high-resolution techniques capable of real-time dynamic analysis.
Future Directions
- AI-Driven Spectral Analysis: As receptor NMR data sets grow in size and complexity, so does the need for intelligent analysis tools. Machine learning and AI algorithms are now being trained to deconvolute overlapping peaks, predict chemical shift perturbations (CSPs), and detect patterns or allosteric networks hidden in high-dimensional data sets.
- Time-Resolved NMR: Understanding receptor function means watching it happen—in real time. Emerging microfluidic devices, such as rapid mixing systems, allow researchers to introduce ligands or cofactors into NMR samples on millisecond timescales, or to follow binding kinetics, conformational transitions, or intermediate states in action.
- In-Tissue NMR: Development of non-invasive NMR-active probes, such as 19F-labeled ligands or hyperpolarized metabolites, that selectively bind to receptors in living tissue. Development of non-invasive NMR-active probes, such as 19F -labeled ligands or hyperpolarized metabolites, that selectively bind to receptors in living tissue.
In conclusion, NMR spectroscopy provides a dynamic, atomic-level lens into receptor behavior, bridging structural biology and pharmacology. By focusing on receptor conformation, dynamics, and environmental interactions, NMR informs rational drug design, mechanism-of-action studies, and personalized medicine. As sensitivity and computational integration improve, NMR will remain pivotal in decoding complex receptor biology.
Creative Biostructure offers high-quality customized NMR spectroscopy services, contact us today to learn how our NMR services can optimize your receptor studies.
References
- Amaral M, Kokh DB, Bomke J, et al. Protein conformational flexibility modulates kinetics and thermodynamics of drug binding. Nat Commun. 2017;8(1):2276. https://doi.org/10.1038/s41467-017-02258-w
- Dubey A, Takeuchi K, Reibarkh M, Arthanari H. The role of NMR in leveraging dynamics and entropy in drug design. J Biomol NMR. 2020;74(10-11):479-498. https://doi.org/10.1007/s10858-020-00335-9
- Ge H, Wang H, Pan B, et al. G protein-coupled receptor (GPCR) reconstitution and labeling for solution nuclear magnetic resonance (NMR) studies of the structural basis of transmembrane signaling. Molecules. 2022;27(9):2658. https://doi.org/10.3390/molecules27092658
- Howarth GS, McDermott AE. High-resolution magic angle spinning NMR of KcsA in liposomes: the highly mobile c-terminus. Biomolecules. 2022;12(8):1122. https://doi.org/10.3390/biom12081122
- Karamanos TK, Kalverda AP, Thompson GS, Radford SE. Mechanisms of amyloid formation revealed by solution NMR. Progress in Nuclear Magnetic Resonance Spectroscopy. 2015;88-89:86-104. https://doi.org/10.1016/j.pnmrs.2015.05.002
- Kumar A, Narayanan V, Sekhar A. Characterizing post-translational modifications and their effects on protein conformation using NMR spectroscopy. Biochemistry. 2020;59(1):57-73. https://doi.org/10.1021/acs.biochem.9b00827
- Luchinat E, Banci L. In-cell NMR: recent progresses and future challenges. Rend Fis Acc Lincei. 2023;34(3):653-661. https://doi.org/10.1007/s12210-023-01168-y
- Neidle S. Methods for studying nucleic acid structure. In: Principles of Nucleic Acid Structure. Elsevier; 2008:1-19. https://doi.org/10.1016/B978-012369507-9.50002-9
- Yang S. BPS2025 - Integrative biophysics of allosteric interactions and disruption in estrogen receptor. Biophysical Journal. 2025;124(3):59a. https://doi.org/10.1016/j.bpj.2024.11.388
- Zhang H, Gong W, Wu S, Perrett S. Studying protein folding in health and disease using biophysical approaches. Bose K, ed. Emerging Topics in Life Sciences. 2021;5(1):29-38. https://doi.org/10.1042/ETLS20200317