Isotopic Analysis by NMR and Applications for API Traceability
In terms of its economic impact and the safety of patient groups, drug counterfeiting is a major problem. Therefore, a wide range of technologies and methods have been used in the fight against counterfeit drugs. In addition to the appearance problems that are relatively easy to detect (the packaging, shape, and color of tablets), a set of analytical tools are also needed to verify the drug itself.
The measurement of isotope ratio (irm-NMR) by nuclear magnetic resonance is recognized as a powerful technique for evaluating the traceability of active pharmaceutical ingredients (API). The results obtained by this method are extremely valuable for detecting many different types of counterfeit products.
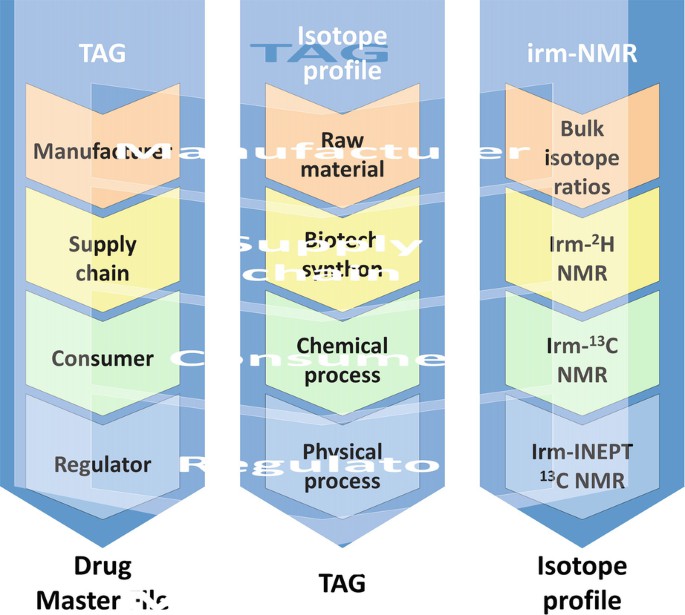 Figure 1. The intimate pedigree of the molecule (API) is built up from an isotope profile that can be the collection of data obtained by irm-MS and irm-NMR. (Remaud & Akoka, 2018)
Figure 1. The intimate pedigree of the molecule (API) is built up from an isotope profile that can be the collection of data obtained by irm-MS and irm-NMR. (Remaud & Akoka, 2018)
As an expert in the field of nuclear magnetic resonance, the irm-NMR technology of Creative Biostructure can be used for the analysis of active drug components. The drug counterfeiting behaviors that can be detected by this set of analysis tools include
- Use of synthetic ingredients when marking natural sources.
- The process of intentional duplication.
- Patent infringement of existing generic drug patents.
- Products produced in non-specific countries of origin (circumvention of anti-dumping duties or other duties).
- Diversion (e.g., re-importation).
Technology Principles
NMR spectroscopy is the only analytical technique to quantify each isotope of a given molecule. In fact, irm-NMR is also known as 2H-SNIF-NMR (natural isotope fractionation at a specific location studied by nuclear magnetic resonance). This method opens up a direct way to obtain natural isotope fractionation at a specific location, which is a powerful technology to verify the source of commercial products.
General principles
The principle of SNIF-NMR is based on "natural isotope fractionation". To ensure the authenticity of the API, two cores are usually used:
Hydrogen nucleus: 2H-SNIF-NMR method is the initial application of SNIF-NMR, which measures the ratio of deuterium/hydrogen at each position of the sample molecule.
Carbon nucleus: 13C-NIF-NMR method opens up new possibilities (new molecules and new applications) for SNIF-NMR analysis. This method will use a ratio of 13C to 12C at each site of the molecule.
Steps of the Service
- Standardized preparation of NMR samples.
- NMR acquisition.
- Result interpretation and authenticity report.
Our Technology
- Irm- 2H NMR.
- One-pulse irm-13C NMR.
- Multi-pulse irm-13C NMR.
Applications
API pedigree can be defined by establishing isotope fingerprints, such as specific labels or barcodes, which can
- Provide the manufacturer with a unique mark for the whole process of manufacturing API.
- And as a tool to check the authenticity of API according to its declaration source.
- Enhance supply chain security from the manufacturing plant to the pharmacy.
- Help patients have confidence in the drugs they use.
Creative Biostructure is committed to providing high-quality NMR analysis services to advance the life sciences fields. If you have any questions or needs, please contact us and our customer service staff will help you the first time.
Ordering Process
Reference
- Remaud, G. S., & Akoka, S. Isotope ratio monitoring by NMR: Part 3 – New applications for traceability of active pharmaceutical ingredients. Modern Magnetic Resonance. 2018, 2233-2251.
