In the complex ecosystem of the immune system, B cells play a central role in initiating humoral immune responses. They achieve this by specifically recognizing particular regions on antigen molecules through their surface B cell receptors (BCRs). This recognition triggers B cell activation, proliferation, and differentiation into plasma cells, which ultimately produce and secrete large quantities of highly specific antibodies. These antibodies subsequently bind to specific sites on the antigen, known as antibody epitopes, to neutralize pathogens or toxins.
This article reviews B cell epitope analysis, including their definition, importance, computational prediction, mapping techniques, and applications in biomedical research.
What Is a B Cell Epitope
A B cell epitope is a specific region on an antigen molecule that is recognized and bound by a BCR. This interaction initiates the humoral immune response by activating B cells.
B cell recognition of epitopes is highly specific, akin to the fit between a lock and key. A single antigen molecule may contain multiple distinct B cell epitopes, capable of being recognized by different B cell clones, thereby eliciting a diverse immune response.
Understanding B cell epitopes is fundamentally important because it directly relates to:
- Initiation of Humoral Immune Response: Recognition of the B cell epitope by the BCR is the signal that activates the B cell, serving as the starting point for antibody production.
- Formation of Immunological Memory: A portion of activated B cells differentiate into memory B cells, providing rapid and robust immune protection upon subsequent encounters with the same antigen.
- Basis of Antibody Diversity: Different B cell clones recognize different B cell epitopes, which is the foundation for generating a diverse library of antibodies capable of binding to various antibody epitopes on the antigen.
Related Reading
B Cell Epitope Properties
B cell epitopes, the antigen regions recognized by BCRs, have properties closely linked to how B cells interact with antigens:
- Recognition Mechanism: BCRs typically recognize the three-dimensional spatial structure of the antigen in its native state. This means that B cell epitopes are often conformational epitopes, formed by residues that are close in the 3D structure but may be distant in the amino acid sequence. These residues form a specific spatial conformation that is recognized and bound by the complementary determining regions (CDRs) of the BCR.
- Linear vs. Conformational: While conformational epitopes are the typical form of B cell epitopes recognized on native antigens by BCRs, in some cases, B cells can also recognize linear epitopes, which consist of a continuous sequence of amino acids. This often occurs after the antigen has been processed by antigen-presenting cells (APCs), and linear peptide fragments are presented and recognized by B cells. However, for intact, unprocessed antigens, the BCR primarily recognizes surface conformational features.
- Accessibility: B cell epitopes must be located on the surface of the antigen, making them accessible for recognition and binding by the cell-surface BCR.
- Flexibility: Appropriate flexibility can potentially facilitate the B cell epitope in adapting to the binding conformation of the BCR.
Upon recognizing a B cell epitope, a series of events occurs, including antigen internalization, processing, presentation of peptide fragments to T cells, and the B cell's own activation, proliferation, and differentiation. Ultimately, the plasma cells differentiated from activated B cells secrete antibodies. The sites that these antibodies subsequently bind to, the antibody epitopes, are often identical or highly overlapping with the original B cell epitopes recognized by the BCR, especially for conformational epitopes. Thus, B cell epitopes largely dictate which regions of the antigen the resulting antibodies will bind to.
 Figure 1. Linear and Conformational B-Cell Epitopes. Schematic showing linear B-cell epitopes formed by continuous amino acids and conformational epitopes formed by non-sequential residues. (Ong Y C, et al., 2024)
Figure 1. Linear and Conformational B-Cell Epitopes. Schematic showing linear B-cell epitopes formed by continuous amino acids and conformational epitopes formed by non-sequential residues. (Ong Y C, et al., 2024)
B Cell Epitopes vs T Cell Epitopes
It is important to distinguish B cell epitopes from T cell epitopes. Unlike B cell epitopes, T cell epitopes are typically linear peptide fragments derived from processed antigens. These peptides are presented on the surface of antigen-presenting cells by major histocompatibility complex (MHC) molecules and recognized by T cell receptors (TCRs). T cell epitopes play a central role in driving cellular immune responses and providing helper signals critical for effective B cell activation.
For a deeper understanding of how T cell epitopes are identified and analyzed, refer to Overview of T Cell Epitope Prediction and Mapping.
Why B Cell Epitope Analysis Matters
B cell epitope analysis is essential because these epitopes are the molecular triggers that initiate the humoral immune response, mediated by B cells and antibodies. Understanding which parts of an antigen are recognized by BCRs is critical for:
- Epitope Based Vaccine Design: Identifying key immunodominant B cell epitopes that are most likely to elicit a strong, protective antibody response is paramount for designing effective subunit vaccines. Targeting conserved B cell epitopes across different strains of a pathogen can lead to broader protection. When designing vaccines based on B cell epitope knowledge, it's vital to consider that antibody responses in vivo may have different outcomes than suggested by simple in vitro binding assays, and in some cases, antibody binding may enhance viral entry through antibody-dependent enhancement (ADE), a factor that must be carefully considered when applying epitope data in vaccine design.
- Diagnostic Development: Understanding the B cell epitopes on pathogens that elicit antibodies allows for the development of highly specific diagnostic tests (like ELISA) that detect antibodies targeting these key sites, indicating exposure or disease status. While the assay detects the antibody epitope, the knowledge originates from studying the B cell epitope response.
- Therapeutic Antibody Development: Therapeutic antibodies, used to treat a wide range of diseases, exert their effects by binding to specific target antigens. Generating or selecting therapeutic antibodies often involves understanding which B cell epitopes could be targeted to initiate a desired immune modulation or neutralization effect, and subsequently mapping the precise antibody epitopes bound by the therapeutic agent is crucial for optimizing its function and specificity.
- Understanding Autoimmune Diseases: In autoimmune diseases, the immune system mistakenly targets self-antigens. Identifying the specific self-B cell epitopes that trigger autoreactive B cells can provide crucial insights into disease mechanisms and potential therapeutic targets aimed at modulating these initial recognition events.
- Allergy Research: Pinpointing the B cell epitopes on allergens responsible for triggering B cell sensitization and subsequent IgE antibody production helps in understanding allergic reactions and developing epitope-specific immunotherapies targeting these initial B cell recognition events.
Computational B Cell Epitope Prediction
Due to the time and resources required for experimental identification, computational B cell epitope prediction has become an essential tool for rapid screening and prioritization. While early prediction methods faced limitations, significant advancements, particularly with machine learning, have greatly improved their utility in B cell epitope prediction.
These in silico methods leverage biological data and computational algorithms to predict the likelihood of a particular region serving as a B cell epitope. They can be broadly categorized:
Sequence-Based Methods
These methods rely solely on the amino acid sequence of the antigen. They often use statistical correlations with known linear epitopes, looking for regions enriched in properties like hydrophilicity, flexibility, or antigenicity. Some b cell epitope prediction tools fall into this category.
Structure-Based Methods
Recognizing the importance of conformational epitopes recognized by BCRs on native antigens, these methods utilize the 3D structure of the antigen. They analyze surface accessibility, spatial proximity of residues, and structural features like turns and loops, which are often found in epitopes. Utilizing protein structure for B cell epitope mapping software often involves these principles for predicting potential interaction sites.
Various B cell epitope prediction tools and B cell epitope mapping software platforms are available online and commercially. These tools employ diverse algorithms to predict regions likely to initiate a B cell response or be bound by antibodies. Examples include tools integrated into resources like the IEDB (e.g., Bepipred for linear prediction, DiscoTope and ElliPro for conformational prediction), as well as standalone tools like SEPPA, EPITOPIA, CBTOPE, EPCES, and PEASE.
Machine Learning and AI
More recent and sophisticated approaches employ machine learning and deep learning algorithms trained on large datasets of known epitopes. These methods can learn complex patterns within sequence and structural data to improve prediction accuracy.
AlphaEpi presents a novel computational framework for improving the prediction of conformational B-cell epitopes. Current methods face two significant challenges: limited availability of experimentally validated structures and difficulties in effectively integrating structural and sequence data.
To address these challenges, AlphaEpi innovatively integrates the latest AlphaFold 3 structural predictions with graph neural networks (GNNs), introducing two key components:
- Dynamic Selector Module: This module dynamically evaluates the reliability of structural and sequence features. When structural predictions are uncertain, it prioritizes evolutionary sequence information derived from ESM-2 embeddings. Conversely, when the structural data are robust, it leans on spatial information, thus adaptively enhancing feature selection and mitigating noise.
- Graph Fusion Module: After preliminary selection, AlphaEpi constructs a weighted graph where each node represents a residue, edges represent structural proximity (from AlphaFold 3's contact probability matrix), and node features stem from sequence embeddings. A multi-layer GNN then integrates these heterogeneous features to accurately predict which residues constitute epitopes.
Experimental validation on a benchmark dataset (BP3C50ID and an external test set) showed that AlphaEpi significantly outperforms state-of-the-art methods like BepiPred-3.0 and GraphBepi, achieving higher AUC scores. Ablation studies further confirmed the critical role of the dynamic selector and the advanced fusion strategy. Notably, while AlphaFold 3 alone provides structural information, it is the dynamic and context-sensitive feature selection combined with graph-based integration that gives AlphaEpi a substantial advantage.
Refer to our article AlphaFold3: Accurate Structure Prediction of Molecular Interactions for more details.
 Figure 2. AlphaEpi Flowchart for B Cell Epitope Prediction. Structural and sequence data are processed through CNN, LSTM, and GNN modules to predict epitope probabilities for each residue. (Jiang F, et al., 2024)
Figure 2. AlphaEpi Flowchart for B Cell Epitope Prediction. Structural and sequence data are processed through CNN, LSTM, and GNN modules to predict epitope probabilities for each residue. (Jiang F, et al., 2024)
It's crucial to emphasize that experimental validation, typically through antibody binding assays or other mapping techniques, is almost always required. Predictions provide probabilities, not certainties, regarding which regions are B cell epitopes or will become antibody epitopes.
Experimental B Cell Epitope Mapping Techniques
Experimentally identifying the precise location and composition of epitopes on an antigen is known as epitope mapping. Given that secreted antibodies are the tools typically used for detection and are a direct product of B cell epitope recognition, most experimental methods are focused on mapping where a specific antibody binds to an antigen (i.e., mapping antibody epitopes). However, mapping these antibody epitopes provides indispensable empirical data that reflects and informs our understanding of the original B cell epitopes that initiated the response. These methods often utilize native antigens, antigen fragments, recombinant proteins, synthetic peptides, or modified antigens. Each method offers unique advantages but may also face challenges, such as definitively distinguishing between discontinuous and linear epitopes.
B Cell Epitope Mapping via X-ray Crystallography
This high-resolution technique involves co-crystallizing antibodies with their antigens. Analyzing the crystal structure provides atomic-level detail of the interaction interface (the antibody epitope), revealing the exact residues involved.
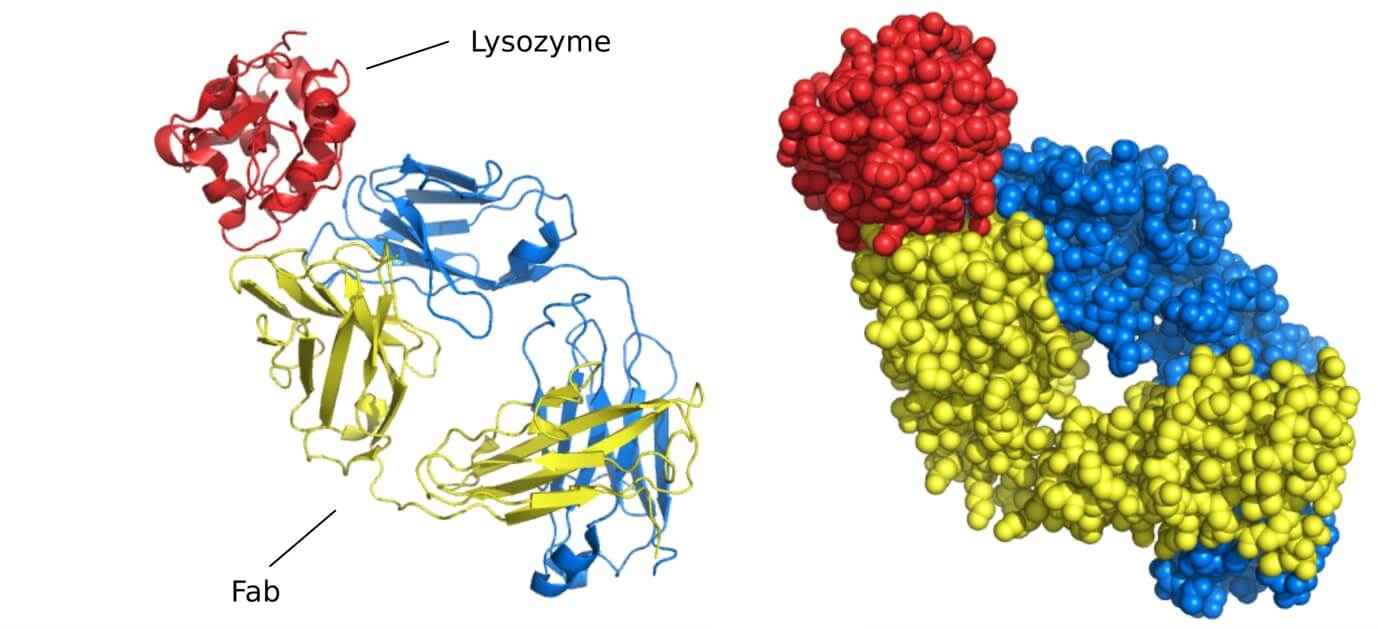
Case Study: Mapping Cetuximab's Target on EGFR
By using X-ray crystallography to determine the 2.8 Å resolution structure of the cetuximab Fab fragment bound to the soluble extracellular region of EGFR (sEGFR), researchers precisely mapped the antibody's binding site, or epitope. The structure revealed that cetuximab attaches specifically to domain III of sEGFR, directly overlapping with where natural growth factors like EGF bind. This detailed map showed how the antibody physically blocks ligand binding by partially occluding the binding region on domain III and also sterically hinders the conformational changes in EGFR required for dimerization and activation.
 Figure 3. Crystal Structure of the FabC225-sEGFR complex. (A) Ribbons representation highlights FabC225 chains and sEGFR domains. (B) Close-up of the binding interface, showing key side-chain interactions and hydrogen bonds between FabC225 and sEGFR. (Li S, et al., 2005)
Figure 3. Crystal Structure of the FabC225-sEGFR complex. (A) Ribbons representation highlights FabC225 chains and sEGFR domains. (B) Close-up of the binding interface, showing key side-chain interactions and hydrogen bonds between FabC225 and sEGFR. (Li S, et al., 2005)
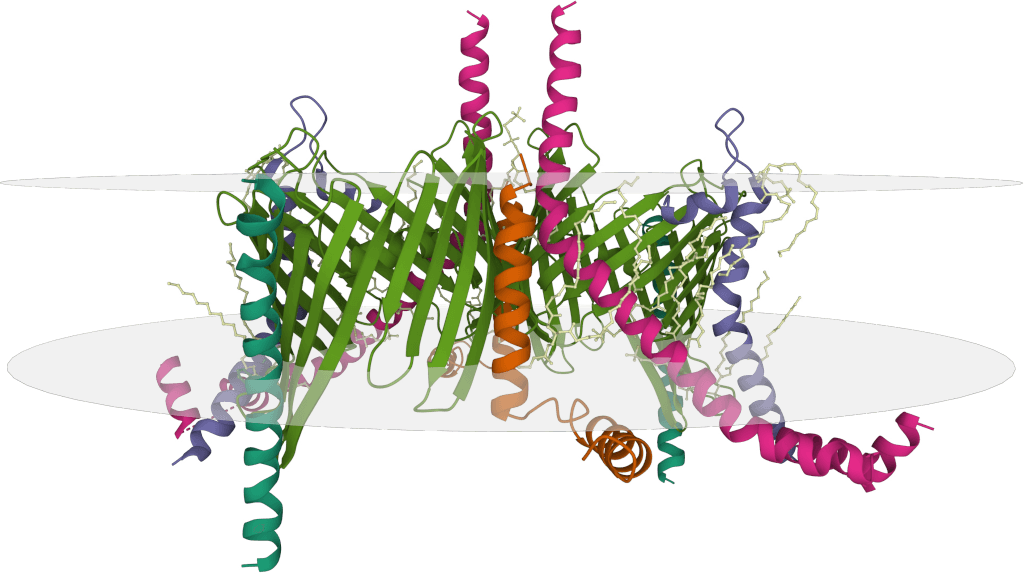
B Cell Epitope Mapping via Cryo-EM
Particularly useful for large or complex antigens, cryo-electron microscopy (Cryo-EM) determines the 3D structure of antibody-antigen complexes at high resolution. This allows for detailed visualization of the antibody epitope on the antigen surface, providing crucial context for understanding the B cell epitope on the native structure.
Case Study: Mapping a Neutralizing Epitope on SARS-CoV-2 Spike SD1
Using cryo-EM, researchers were able to visualize the binding of a neutralizing antibody, P008_60, to the SARS-CoV-2 spike protein. The 4.3 Å resolution Cryo-EM structure of the spike monomer bound to the P008_60 Fab fragment precisely mapped the antibody's epitope to the SD1 domain, specifically detailing extensive interactions with the L3 loop of this subdomain. This was a significant finding because this SD1 epitope is largely hidden or "occluded" in the known structures of the full spike trimer in its prefusion state.
 Figure 4. Characterization of the SD1 Epitope Recognized by P008_60. (A) Cryo-EM structure of the S1-P008_60 Fab complex with S1 in green, Fab light chain in pink, and heavy chain in orange. (B) Close-up of the binding interface showing key residues. (C) SARS-CoV-2 spike trimer with the P008_60 epitope (L3) highlighted in red. (Seow J, et al., 2022)
Figure 4. Characterization of the SD1 Epitope Recognized by P008_60. (A) Cryo-EM structure of the S1-P008_60 Fab complex with S1 in green, Fab light chain in pink, and heavy chain in orange. (B) Close-up of the binding interface showing key residues. (C) SARS-CoV-2 spike trimer with the P008_60 epitope (L3) highlighted in red. (Seow J, et al., 2022)
Mass Spectrometry (MS)-based Methods
Techniques like Hydrogen-deuterium exchange mass spectrometry (HDX-MS) compare antigen dynamics or fragmentation patterns with and without antibody binding. Regions protected by antibody binding highlight potential antibody epitopes, offering insights into the structural regions likely involved in the initial B cell epitope recognition.
HDX-MS provides dynamic insights into how proteins undergo conformational changes upon binding interactions, such as those involving antibodies. When combined with structural imaging techniques like Cryo-EM, HDX-MS offers a more comprehensive understanding of antibody-antigen interactions.
Case Study: Revealing Antibody Binding to the SARS-CoV-2 Spike Using HDX-MS
In a study involving the neutralizing antibody P008_60, which targets the SD1 domain of the SARS-CoV-2 spike protein, Cryo-EM identified the binding site within the SD1 region. HDX-MS complemented these findings by revealing conformational changes associated with antibody engagement.
Specifically, HDX-MS analysis demonstrated that residues within the SD1 region (amino acids 516–585) exhibited reduced deuterium uptake following antibody binding, indicating decreased solvent accessibility and increased structural protection. A measurable shift of approximately 5 Da in deuterium incorporation confirmed direct binding and localized stabilization of the spike protein. Moreover, HDX-MS revealed that antibody binding induced broader conformational changes across the spike, stabilizing some regions while increasing flexibility in others.
These findings illustrate how HDX-MS complements static structural methods, providing dynamic and functional insights into antibody neutralization mechanisms.
 Figure 5. HDX Mapping of Spike Protein Conformational Changes Induced by P008_60. (A) Structural mapping of HDX changes upon P008_60 binding: blue indicates decreased HDX, red indicates increased HDX, and gray indicates no change. (B) Differential deuterium uptake plots showing examples of epitope stabilization and destabilization after binding. (Seow J, et al., 2022)
Figure 5. HDX Mapping of Spike Protein Conformational Changes Induced by P008_60. (A) Structural mapping of HDX changes upon P008_60 binding: blue indicates decreased HDX, red indicates increased HDX, and gray indicates no change. (B) Differential deuterium uptake plots showing examples of epitope stabilization and destabilization after binding. (Seow J, et al., 2022)
Nuclear Magnetic Resonance (NMR)
NMR can probe atomic-level interactions and dynamics at the binding interface. Changes in NMR signals upon antibody binding identify residues in the antibody epitope, providing fine details relevant to the B cell epitope's characteristics.
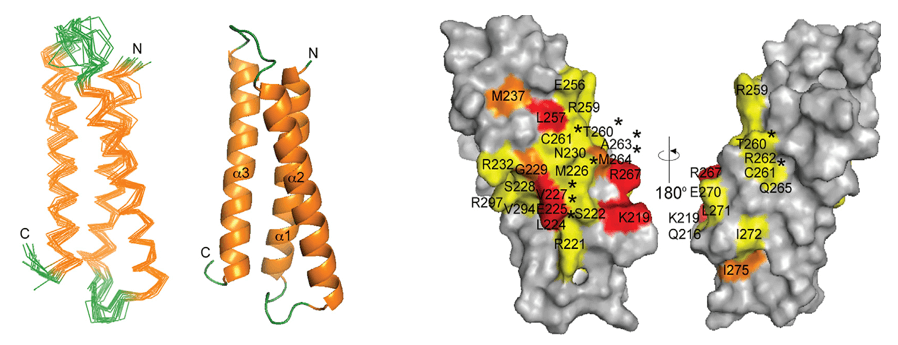
Case Study: Epitope Mapping of Anti-Dengue Antibody DV32.6 Using NMR
In this study, solution NMR spectroscopy was employed to experimentally map the epitope of the human anti-Dengue antibody DV32.6 on domain III (DIII) of each Dengue virus serotype. This was achieved by observing changes in the NMR signals of DIII residues upon antibody binding, which indicated the interaction interface at a residue-specific level. Approximately 20% of the surface residues of DIII showed changes in their NMR signals upon binding DV32.6. The NMR data identified the epitope region centered around residues 306-325, noting slight variations between serotypes and the presence of both conserved and non-conserved residues within this region. The experimental epitope information obtained from NMR was then crucial for guiding and validating computational docking simulations, enabling the selection and refinement of accurate 3D structural models of the antibody-antigen complexes, which were subsequently used for rational antibody engineering.
 Figure 6. NMR Epitope Mapping and Docking Analysis of DV32.6 Binding to Dengue Serotypes. (A, B) NMR mapping identifies affected residues (red) on DIII of each Dengue serotype, showing conserved and variable sites. (C) Computational docking highlights DV32.6 antibody residues involved in binding, with differences in light and heavy chain contributions across serotypes. (Simonelli L, et al., 2013)
Figure 6. NMR Epitope Mapping and Docking Analysis of DV32.6 Binding to Dengue Serotypes. (A, B) NMR mapping identifies affected residues (red) on DIII of each Dengue serotype, showing conserved and variable sites. (C) Computational docking highlights DV32.6 antibody residues involved in binding, with differences in light and heavy chain contributions across serotypes. (Simonelli L, et al., 2013)
Binding Assays (e.g., ELISA, Western Blot, Dot Blot)
These versatile assays test antibody binding to various antigen formats. By using overlapping protein fragments or synthetic peptides spanning the antigen sequence, linear antibody epitopes can be identified. ELISA techniques and Western blots are common. While mapping linear antibody epitopes directly, these methods contribute to understanding the potential repertoire of sites recognized following B cell epitope triggering.
Methods Utilizing Recombinant & Synthetic Antigens
- Peptide Microarray (PMA): This high-throughput method involves synthesizing libraries of peptides and spotting them on a surface. Testing antibody binding to these synthetic peptides is highly effective for mapping linear antibody epitopes. This is a direct method of b cell epitope mapping using synthetic peptides if interpreted as identifying linear sites relevant to the B cell response or antibody binding.
- Surface Plasmon Resonance (SPR): SPR measures real-time binding kinetics between antibodies and antigens. By using different antigen fragments or mutants, SPR can help characterize the binding properties of antibody epitopes, providing functional data related to the initial B cell epitope recognition.
Methods Utilizing Modified Antigens
- Site-Directed Mutagenesis: Systematically altering residues within a suspected epitope and testing antibody binding helps pinpoint critical contact points in the antibody epitope.
Each of these experimental techniques contributes valuable, empirical data to epitope mapping (primarily antibody epitope mapping), crucially informing and validating our understanding of the B cell epitopes that initiate the humoral immune response.
Select Service
Critical Factors Shaping B Cell Epitope Immunogenicity
Identifying the location of a B cell epitope is only part of the process. Equally important is understanding the intrinsic properties of B cell epitopes that determine their immunogenicity, or their ability to effectively trigger a B cell response. Not all regions of an antigen are equally likely to become immunodominant epitopes capable of eliciting strong antibody production. These properties are critical both for the initial recognition by B cell receptors and for the subsequent binding of antibodies to the corresponding antibody epitopes.
Key properties that contribute to a B cell epitope's immunogenicity and subsequent antibody binding affinity include:
- Accessibility and Surface Exposure: A B cell epitope must be physically accessible to the BCR on the native antigen structure. Similarly, the resulting antibody epitope must be accessible to circulating antibodies.
- Conformational Stability and Flexibility: For conformational B cell epitopes, maintaining a stable 3D structure is crucial for consistent BCR recognition. Some flexibility might aid induced fit upon binding by BCRs or antibodies, but overall stability is key for defining the epitope.
- Charge and Hydrophilicity: B cell epitopes often reside in hydrophilic regions on the protein surface, facilitating interaction with the aqueous environment and the binding sites of BCRs and antibodies. Charged residues can play a significant role in specific electrostatic interactions.
- Molecular Context: The surrounding amino acids, post-translational modifications, and the overall structure of the antigen can influence a B cell epitope's conformation and presentation for BCR recognition, which in turn affects the resulting antibody binding.
- Processing and Presentation (for T-dependent responses): While B cells recognize intact B cell epitopes, the magnitude of the antibody response to many antigens is boosted by T helper cell help, which recognizes peptide epitopes presented by MHC. The context of T cell help influences the overall B cell epitope driven response.
- Influence of Adjuvants: Adjuvants used in vaccines can significantly influence which B cell epitopes become immunodominant by affecting antigen uptake, processing, presentation, and immune cell activation.
Analyzing these properties in conjunction with epitope mapping and B cell epitope prediction helps in designing more effective vaccines and therapeutic antibodies by focusing on B cell epitopes most likely to initiate a desired, robust humoral immune response.
At Creative Biostructure, we specialize in providing comprehensive B cell epitope prediction and experimental epitope mapping services to accelerate your immunology research. Whether you require computational prediction, high-resolution structural mapping, or customized visualization of B cell epitopes, our expert team is ready to assist. Contact us to discuss how we can support your project needs!
References
- Li S, Schmitz K R, Jeffrey P D, et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005, 7(4): 301-311. https://doi.org/10.1016/j.ccr.2005.03.003.
- Simonelli L, Pedotti M, Beltramello M, et al. Rational engineering of a human anti-dengue antibody through experimentally validated computational docking. PloS one. 2013, 8(2): e55561. https://doi.org/10.1371/journal.pone.0055561
- Seow J, Khan H, Rosa A, et al. A neutralizing epitope on the SD1 domain of SARS-CoV-2 spike targeted following infection and vaccination. Cell Reports. 2022, 40(8). https://doi.org/10.1016/j.celrep.2022.111276
- Hamed S M, Sakr M M, El-Housseiny G S, et al. State of the art in epitope mapping and opportunities in COVID-19. Future science OA. 2023, 9(1): FSO832. https://doi.org/10.2144/fsoa-2022-0048
- Ong Y C, Tejo B A, Yap W B. An Immunoinformatic Approach for Identifying and Designing Conserved Multi-Epitope Vaccines for Coronaviruses. Biomedicines. 2024, 12(11): 2530. https://doi.org/10.3390/biomedicines12112530
- Jiang F, Guo Y, Ma H, et al. AlphaEpi: Enhancing B Cell Epitope Prediction with AlphaFold 3. Proceedings of the 15th ACM International Conference on Bioinformatics. Computational Biology and Health Informatics. 2024: 1-8. https://doi.org/10.1145/3698587.370138