The immune system's ability to specifically recognize and respond to foreign or abnormal entities depends on the precise interaction between immune receptors and defined regions on target molecules, known as epitopes. In B cells, this recognition is mediated by antibodies that bind directly to conformational or linear epitopes on antigens. In T cells, recognition occurs through T cell receptors (TCRs) binding to short linear peptide epitopes derived from processed antigens and presented on the cell surface by major histocompatibility complex (MHC) molecules.
What Is Epitope Mapping?
Epitope mapping is the process of experimentally identifying and characterizing the specific regions on antigens that are recognized by B cell receptors, T cell receptors, or antibodies. It is a critical technique across immunology, vaccine development, therapeutic design, and diagnostics.
In immunology, epitope mapping helps reveal the molecular targets that drive protective immunity, autoimmune diseases, and allergic responses. In vaccine development, identifying immunodominant B cell epitopes and T cell epitopes is essential for designing vaccines that elicit strong humoral and cellular immunity. In therapeutics, B cell epitope mapping guides the development of monoclonal antibodies, while T cell epitope identification underpins the creation of cellular therapies such as CAR-T and TCR-T. In diagnostics, epitope characterization enables the development of sensitive assays for detecting infections, autoimmune conditions, and immune status.
The diverse nature of epitopes (linear vs. conformational for B cells; different peptide sequences binding to various MHC alleles for T cells) necessitates a wide array of mapping techniques. Selecting the most appropriate method, or often a combination, is vital for generating accurate, relevant, and impactful data.
Understanding Epitope Types for Mapping
Effective epitope mapping begins with a clear understanding of the distinct characteristics of epitopes recognized by B cells and T cells. An epitope, or antigenic determinant, refers to the specific molecular structure on an antigen that interacts with the antigen-binding site of an antibody or a TCR via an MHC molecule.
B cell epitopes are recognized directly by B cell receptors (BCRs) or secreted antibodies. They can be composed of amino acids, carbohydrates, lipids, or nucleic acids, and generally fall into two categories:
- Linear epitopes, formed by continuous sequences of amino acids and recognized based on their primary structure.
- Conformational epitopes, formed by discontinuous residues brought together through three-dimensional protein folding, recognized based on the protein's tertiary or quaternary structure. B cell epitopes typically span 5-15 amino acids or 3-4 saccharide units.
 Figure 1. B-Cell Epitope Recognition and Antibody-Antigen Interaction. (Sanchez-Trincado J L, et al., 2017)
Figure 1. B-Cell Epitope Recognition and Antibody-Antigen Interaction. (Sanchez-Trincado J L, et al., 2017)
T cell epitopes are short, linear peptide fragments derived from intracellular processing of proteins and recognized by TCRs only when presented on the surface of antigen-presenting cells (APCs) via MHC molecules. Based on the presenting MHC type, T cell epitopes are classified as:
- CD8+ T cell epitopes, typically 8-11 amino acids long and presented by MHC Class I molecules on most nucleated cells, often derived from intracellular or viral proteins.
- CD4+ T cell epitopes, typically 13-25 amino acids long and presented by MHC Class II molecules, commonly found on professional APCs such as dendritic cells, macrophages, and B cells, usually derived from extracellular or pathogen proteins.
The TCR recognizes the combined structure of the peptide and the MHC molecule. Thus, the core principle of epitope mapping is to identify the specific residues or structural features that form the binding interface for the antibody or TCR.
 Figure 2. T-Cell Epitope Recognition via MHC I and II Pathways. (Sanchez-Trincado J L, et al., 2017)
Figure 2. T-Cell Epitope Recognition via MHC I and II Pathways. (Sanchez-Trincado J L, et al., 2017)
Overview of Epitope Mapping Techniques
The methodologies employed for epitope mapping differ significantly depending on whether the target is a B-cell or T-cell epitope, reflecting their distinct recognition mechanisms.
Techniques can be broadly categorized, often with specific applications for B-cells, T-cells, or sometimes informing both:
- Peptide-Based Methods: Primarily for identifying linear B-cell epitopes and potential T-cell peptide binders.
- Functional Assays: For B-cells (e.g., Mutagenesis-based) identifying critical binding residues; for T-cells (e.g., cell-based assays) identifying immunogenic peptides.
- Display Technologies: Primarily for identifying B-cell peptide/protein binders or mimics.
- Mass Spectrometry-Based Methods: For B-cells (HDX-MS, XL-MS, LiP-MS) mapping binding interfaces; for T-cells (Immunopeptidomics) identifying naturally presented peptides.
- Structure-Based Methods: Primarily for high-resolution mapping of B-cell conformational epitopes (Antibody-Antigen complexes) and T-cell recognition (TCR-pMHC complexes, though less common for de novo mapping).
The choice of technique is influenced by factors such as the type of epitope, the antigen source and characteristics, the desired resolution and throughput, available sample material (purified protein vs. cells vs. patient samples), and the overall project goals.
Peptide-Based Epitope Mapping Approaches
Peptide Array Epitope Mapping / Peptide Scanning
Principle: Libraries of overlapping linear peptides spanning the antigen sequence are synthesized and immobilized on a solid support.
Focus: Identifies continuous amino acid sequences recognized by antibodies (linear B-cell epitopes). Can also be used to screen potential T-cell peptide epitopes for binding to MHC molecules in vitro or stimulating T cells in in vitro assays (though this transitions into functional or binding assays).
Workflow Overview: Involves antigen sequence definition, peptide design (length, overlap), synthesis on membrane/glass, incubation with antibody (for B-cell) or MHC/T cells (for T-cell), washing, and detection (labeled secondary antibody for B-cell; specific detection for MHC binding or T-cell activation).
Pros: High-throughput, cost-effective for identifying linear sequences. Useful starting point for both B and T epitope discovery.
Cons: For B cell epitopes, these methods cannot map conformational epitopes and carry a risk of false positives or negatives. For T cell epitopes, they identify potential binders or in vitro stimulators but do not guarantee proper in vivo antigen processing or actual immunogenicity.
Epitope Mapping ELISA
Principle: Utilizes peptides or protein fragments coated onto ELISA plates.
Application: Screening and confirmation of antibody binding to specific linear sequences or fragments (linear B-cell epitopes). Can also be used to test peptide binding to soluble MHC molecules in vitro.
Relation to peptide arrays/scanning: Often used for validation of hits from array screens or for targeted testing of specific sequences.
Mutagenesis-Based Epitope Mapping Techniques
These methods focus on altering the antigen itself to identify residues critical for antibody binding.
Alanine Scanning Epitope Mapping
Principle: Systematic replacement of individual amino acids with alanine in the full-length antigen protein.
Focus: Identifying specific residues critical for B-cell epitope binding, applicable to both linear and conformational epitopes if applied to the folded protein.
Pros: Identifies functionally important residues. Can provide high resolution for critical contact points.
Cons: Labor-intensive and time-consuming, especially for comprehensive scans. May disrupt protein structure or expression. Less suitable for T-cell epitopes as processing and presentation by MHC is required.
Shotgun Mutagenesis Epitope Mapping
Principle: High-throughput creation and screening of antigen variants with random or targeted mutations expressed on a cell surface.
Focus: High-resolution mapping of functional B-cell epitopes, particularly powerful for conformational epitopes on complex or membrane proteins.
Pros: Comprehensive surface scanning. Highly effective for conformational epitopes.
Cons: Requires robust cell expression and screening systems. Complex library construction. Not applicable for T-cell epitope mapping.
Display Technologies for Epitope Mapping
These technologies use biological systems to present peptide or protein libraries.
Phage Display Epitope Mapping
Principle: Displaying random peptide or protein fragment libraries on phage surfaces, followed by selection for binders to an immobilized antibody.
Focus: Identifies peptide sequences that bind the antibody, representing linear B-cell epitopes or peptides that mimic conformational B-cell epitopes.
Pros: Powerful selection from large libraries. Can identify unexpected binders.
Cons: Identified peptides may only be mimics, not the native epitope. Selection bias can occur. Primarily for B-cell epitope discovery.
Mass Spectrometry in Epitope Mapping
Mass spectrometry offers diverse tools for probing biomolecular interactions and composition.
Hydrogen-Deuterium Exchange MS (HDX-MS)
Principle: Measures changes in solvent accessibility of protein regions upon antibody binding by monitoring deuterium uptake. Protected regions indicate the binding interface.
Focus: Maps conformational B-cell epitopes by identifying regions of the antigen protected by antibody binding.
Pros: Provides structural information on conformational epitopes and dynamics in solution. No crystallization needed.
Cons: Resolution typically peptide-level. Requires specialized equipment and expertise. Less direct for T-cell epitopes, although could potentially inform on conformational changes affecting processing.
Cross-linking Mass Spectrometry (XL-MS)
Principle: Uses chemical cross-linkers to trap proximal residues in the antibody-antigen complex. MS identifies the cross-linked peptides, providing distance constraints between antibody and antigen residues.
Focus: Maps conformational B-cell epitopes by identifying proximal residues at the interaction interface.
Pros: Provides spatial information on the binding interface. Applicable to large complexes.
Cons: Requires optimizing cross-linking conditions. Analysis of cross-linked peptides is complex. Primarily for B-cell epitopes.
Limited Proteolysis MS (LiP-MS)
Principle: Detects changes in proteolytic cleavage patterns of the antigen upon antibody binding, indicating protected regions.
Focus: Identifies regions of the antigen involved in antibody binding (linear or conformational B-cell epitopes), based on altered protease accessibility.
Pros: Relatively simple principle. Can indicate conformational changes.
Cons: Lower resolution compared to HDX-MS or structural methods. Influenced by digestion conditions. Primarily for B-cell epitopes.
Mass Spectrometry-based Immunopeptidomics (MHC-Associated Peptide Proteomics - MAPs)
Principle: Involves isolating MHC Class I or Class II molecules from cells or tissues, eluting the peptides naturally bound in their groove, and identifying the sequences of these peptides using high-resolution MS.
Focus: Identifies naturally processed and presented peptides that are potential T-cell epitopes (both CD4+ and CD8+), reflecting the in vivo peptide repertoire presented by specific MHC alleles.
Pros: Identifies peptides that are actually presented by MHC in situ. Provides a comprehensive list of peptides available for T-cell recognition.
Cons: Technically demanding, requires significant cell/tissue input. Identifying immunogenic peptides requires correlating with functional T-cell data. Does not directly map the TCR contact sites, only the presented peptides.
Functional and Binding Assays
These methods directly assess the ability of peptides to bind MHC or stimulate a T-cell response.
Peptide-MHC Binding Assays
Principle: Measures the affinity of synthetic peptides for specific recombinant MHC molecules in vitro. Techniques include ELISA-based assays, surface plasmon resonance (SPR), or bio-layer interferometry (BLI).
Focus: Screens large numbers of peptides to identify those with high binding affinity to specific MHC alleles. These are considered potential T-cell epitopes based on MHC binding capacity.
Pros: Relatively high-throughput screening for MHC binders. Provides quantitative affinity data.
Cons: In vitro binding does not guarantee cellular processing, presentation, or immunogenicity in vivo. Does not measure actual T-cell activation.
Select Service
Cell-Based T-cell Assays (ELISpot, Intracellular Cytokine Staining (ICS), Proliferation)
Principle: T cells isolated from individuals (e.g., vaccinated, infected, or with disease) are incubated with pools or individual synthetic peptides. T-cell activation is measured by detecting markers like cytokine secretion (ELISpot, ICS), or proliferation (incorporation of labeled nucleotides or dye dilution).
Focus: Identifies immunogenic T cell epitopes by detecting peptides that elicit a measurable T cell response from a specific donor. It can also distinguish between CD4+ and CD8+ T cell responses, for example, through intracellular cytokine staining (ICS) or cell sorting.
Pros: Directly measures functional T-cell responses. Identifies immunodominant epitopes in a population or individual.
Cons: Requires access to live T cells. Throughput can be limited for large peptide libraries. Does not directly identify the restricting MHC molecule without further experimentation. Results can vary based on donor genetics and immune history.
MHC-Peptide Multimer Staining
Principle: Uses fluorescently labeled soluble MHC molecules folded around a specific peptide (multimers, e.g., tetramers, pentamers) to directly bind and identify T cells with TCRs specific for that particular peptide-MHC complex using flow cytometry.
Focus: Quantifies and phenotypically characterizes T cells specific for a defined T-cell epitope presented by a specific MHC allele. This is often used to track responses after an epitope has been identified by other means.
Pros: Directly identifies and quantifies antigen-specific T cell populations. MHC-restricted.
Cons: Requires prior knowledge of the specific peptide and its restricting MHC allele. Limited to the availability of stable, correctly folded peptide-MHC multimers. More a characterization tool than a de novo mapping method.
High-Resolution Structural Epitope Mapping Methods
These techniques provide atomic-level views of interactions.
Epitope Mapping via Cryo-Electron Microscopy (Cryo-EM)
Principle: Direct visualization of frozen protein complexes (Antibody-Antigen or TCR-pMHC) at near-atomic resolution using electron microscopy and 3D reconstruction.
Focus: Direct visualization of conformational B-cell epitopes or the overall structure of the TCR-pMHC complex and its interaction interface. Excellent for large complexes.
Pros: No crystallization required. Suitable for large or flexible complexes. Provides direct visual evidence of the interaction.
Cons: Requires stable complex formation. Resolution can vary. While useful for TCR-pMHC structure, less suitable for de novo identification of presented peptides.
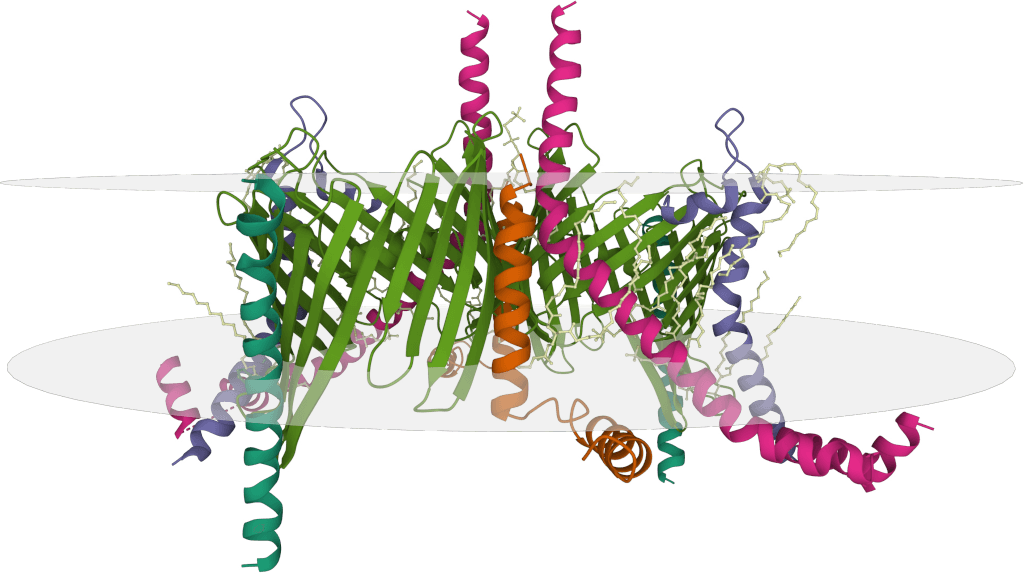
Case Study: Structural Insights into HER2-trastuzumab-pertuzumab Complex via Cryo-EM
In a study using Cryo-EM, the structure of the HER2-trastuzumab-pertuzumab complex was resolved at 4.36 Å, revealing that both antibodies bind independently to distinct subdomains of HER2 without inducing significant conformational changes or cooperative binding. These findings explain why clinical synergy arises from complementary inhibition rather than enhanced affinity and provide crucial insights for designing more effective bispecific molecules with improved flexibility or optimized linker strategies to achieve higher therapeutic efficacy.
 Figure 3. Cryo-EM Structure of HER2-Trastuzumab-Pertuzumab Complex. (A) Cryo-EM map of the HER2-trastuzumab-pertuzumab ternary complex. (B) Local resolution analysis of the reconstruction. (C) Final model fitted into the map, showing HER2 (sky blue), trastuzumab Fab (pink), pertuzumab Fab (orange), and HER2 glycans as sticks. (Hao Y, et al., 2019)
Figure 3. Cryo-EM Structure of HER2-Trastuzumab-Pertuzumab Complex. (A) Cryo-EM map of the HER2-trastuzumab-pertuzumab ternary complex. (B) Local resolution analysis of the reconstruction. (C) Final model fitted into the map, showing HER2 (sky blue), trastuzumab Fab (pink), pertuzumab Fab (orange), and HER2 glycans as sticks. (Hao Y, et al., 2019)
Epitope Mapping through X-ray Crystallography
Principle: Determining the atomic structure of crystallized protein complexes (Antibody-Antigen or TCR-pMHC) using X-ray diffraction.
Pros: Can provide the highest resolution atomic details of the interaction interface.
Cons: Requires successful crystallization, often a major bottleneck. Provides a static picture. Crystallizing TCR-pMHC complexes can be challenging. Like Cryo-EM, used more for characterizing known interactions than de novo mapping of epitopes.
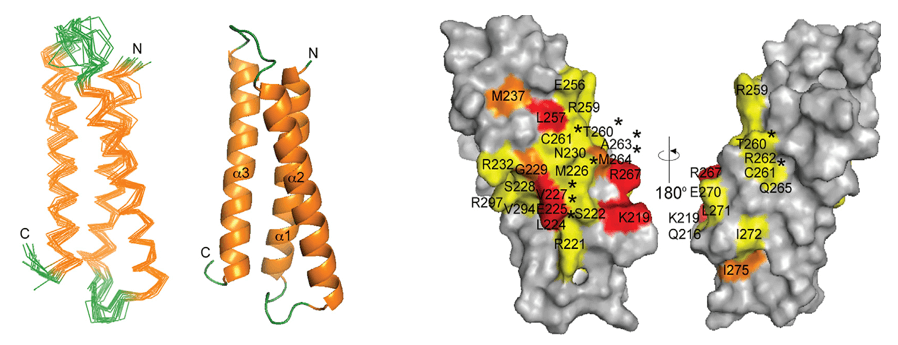
Case Study: Structural Insights into 4-1BB Antibody Binding
Using X-ray crystallography, researchers resolved the structures of human 4-1BB in complex with its ligand 4-1BBL and with two clinical antibodies: utomilumab and urelumab. The study revealed that utomilumab binds between CRDs 3 and 4, blocking ligand access, while urelumab binds to CRD1, allowing simultaneous ligand binding and receptor clustering.
These distinct epitopes explain the functional differences: utomilumab shows weaker but safer immune activation, whereas urelumab induces stronger responses with higher toxicity. This case highlights how structural epitope mapping informs antibody design and therapeutic strategy.
 Figure 4. 4-1BB Binding to Utomilumab and Urelumab Fabs. (A) Utomilumab Fab binds to 4-1BB at the CRD-3/CRD-4 junction. (B) Urelumab Fab binds to the N-terminal region of CRD-1 on 4-1BB. (Chin S M, et al., 2018)
Figure 4. 4-1BB Binding to Utomilumab and Urelumab Fabs. (A) Utomilumab Fab binds to 4-1BB at the CRD-3/CRD-4 junction. (B) Urelumab Fab binds to the N-terminal region of CRD-1 on 4-1BB. (Chin S M, et al., 2018)
Epitope Mapping with Nuclear Magnetic Resonance (NMR)
Principle: Detects changes in the environment of atomic nuclei (chemical shift perturbations) in the antigen upon binding to the antibody or in the peptide/MHC upon binding to the TCR.
Focus: Maps conformational B-cell epitopes or TCR contact residues on the pMHC complex in solution at high resolution. Also provides dynamic information.
Pros: Atomic-level detail in solution. Maps conformational interfaces. Can study dynamics.
Cons: Size limitations for proteins/complexes. Requires isotope labeling. Mapping TCR-pMHC interactions by NMR is very challenging due to size and complexity.
Comparing Epitope Mapping Methods
Selecting the appropriate epitope mapping strategy depends heavily on whether you are targeting the specific type of epitope, the nature of the antigen, the desired level of detail, and available resources. Often, a multi-pronged approach combining different techniques is most effective, leveraging the strengths of each to provide a comprehensive understanding.
| Method | Epitope Type Target | Resolution | Throughput | Sample Requirements | Cost & Time | Advantages | Limitations |
|---|---|---|---|---|---|---|---|
| Peptide Array | B (Linear), T (Potential) | Low-Medium (Sequence) | High | Antigen sequence (for synthesis), Antibody/MHC/Cells | Relatively Low | High-throughput, identifies linear sequences/potential binders | B: Cannot map conformational. T: Doesn't guarantee immunogenicity/processing. |
| Epitope Mapping ELISA | B (Linear), T (Potential) | Low-Medium (Sequence) | Medium-High | Peptides/fragments, Antibody/MHC | Low-Medium | Flexible format, good for validation/screening | B: Primarily linear. T: In vitro binding only. |
| Alanine Scanning | B (Functional) | High (Residue) | Low-Medium | Expressible antigen, Antibody | High (Labor) | Identifies functionally critical B-cell residues | Labor-intensive. Not for T-cell epitopes (processing dependency). |
| Shotgun Mutagenesis | B (Functional) | High (Residue) | High | Expressible antigen (cells), Antibody | High (Setup) | High-resolution functional map, especially for conformational B-cell on cells | Requires cell system. Not for T-cell epitopes. |
| Phage Display | B (Linear/Mimic) | Low-Medium (Sequence) | High | DNA for library, Antibody | Medium | Powerful selection for antibody binders | Peptides may be mimics. Selection bias. Only for B-cell. |
| HDX-MS | B (Conformational) | Medium (Peptide) | Medium | Purified antigen, Antibody | High | Maps conformational B-cell epitopes & dynamics | Peptide-level resolution. Requires MS expertise. Not direct for T-cell. |
| XL-MS | B (Conformational) | Medium (Residue Proximity) | Medium | Purified antigen, Antibody | High | Maps conformational B-cell epitopes via spatial constraints | Complex analysis. Requires cross-linker optimization. Not direct for T-cell. |
| LiP-MS | B (Linear/Conformational) | Low-Medium (Region) | Medium | Purified antigen, Antibody | Medium-High | Relatively simple principle for B-cell protection | Low resolution. Influenced by proteolysis. Not for T-cell. |
| Immunopeptidomics (MAPs) | T (CD4/CD8 - Presented) | High (Peptide Sequence) | Low-Medium | Large cell/tissue input | Very High | Identifies naturally processed & presented T-cell peptide candidates | Technically demanding. Doesn't prove immunogenicity. Requires large sample. |
| Peptide-MHC Binding | T (Potential Binder) | Medium (Affinity) | High | Peptides, Recombinant MHC | Medium | High-throughput screening for MHC binding affinity | In vitro only. Doesn't guarantee immunogenicity/processing. |
| Cell-Based T-cell Assays | T (CD4/CD8 - Immunogenic) | Low-Medium (Peptide Pool) | Low-Medium | Live T cells, Peptides | Medium-High | Directly identifies immunogenic T-cell epitopes in a donor | Requires live cells. Lower throughput for discovery. Doesn't directly give MHC restriction. |
| MHC-Peptide Multimer | T (CD4/CD8 - Specific) | N/A (Tracking) | Medium-High | Live T cells, Pre-made multimers | Medium-High (Multimer Cost) | Direct quantification/phenotyping of specific T cells (known epitope) | Requires known epitope & MHC. Not for de novo mapping. Multimer production can be challenging/costly. |
| Cryo-EM | B (Conformational), T (pMHC-TCR) | High (Near-Atomic) | Low-Medium | Purified complex | Very High | Visualizes large complexes, no crystallization needed (B-cell). pMHC-TCR structure | Requires stable complex/expertise/equipment. Not for de novo mapping of T-cell peptides. |
| X-ray Crystallography | B (Conformational), T (pMHC-TCR) | Very High (Atomic) | Low | Purified, crystallizable complex | Very High | Highest resolution structural detail | Requires crystallization. Static picture. Not for de novo mapping of T-cell peptides. |
| NMR | B (Conformational), T (Contact) | High (Atomic) | Low | Purified, labeled protein/complex | Very High | Atomic detail in solution, dynamics (B-cell). High resolution for pMHC-TCR | Size limit, labeling/expertise, high cost. Less suitable for de novo mapping. |
Choosing the Right Epitope Mapping Method
Selecting the optimal approach requires a clear understanding of the research question and the nature of the target epitope:
- Are you mapping B cell or T cell epitopes? This is the most fundamental distinction determining the primary set of applicable methods.
- For B cell epitopes: Is it likely linear or conformational? What resolution is needed? Is a high-throughput scan required?
- For T cell epitopes: What MHC alleles are relevant? Do you need to identify peptides that bind MHC, or peptides that stimulate T cells? Do you want to identify naturally presented epitopes?
- What resources are available? Consider budget, required equipment (MS, NMR, Cryo-EM, flow cytometry), and technical expertise.
Often, integrating in silico epitope prediction with experimental validation provides the most efficient path, narrowing down the experimental scope. Furthermore, combining different experimental techniques (e.g., peptide screening and structural analysis for B cells, or peptide binding and functional assays for T cells) can provide a more complete and validated understanding of the epitope landscape.
Comparing epitope mapping methods allows researchers to select the most effective techniques for their specific immunology, therapeutic, and diagnostic goals. At Creative Biostructure, we offer tailored B cell and T cell epitope mapping services, guiding you from method selection to experimental validation. Contact us to advance your research with precision.
References
- Abbott W M, Damschroder M M, Lowe D C. Current approaches to fine mapping of antigen-antibody interactions. Immunology. 2014, 142(4): 526-535. https://doi.org/10.1111/imm.12284
- Davidson E, Doranz B J. A high‐throughput shotgun mutagenesis approach to mapping B‐cell antibody epitopes. Immunology. 2014, 143(1): 13-20. https://doi.org/10.1111/imm.12323
- Potocnakova L, Bhide M, Pulzova L B. An introduction to B‐cell epitope mapping and in silico epitope prediction. Journal of Immunology Research. 2016, 2016(1): 6760830. https://doi.org/10.1155/2016/6760830
- Sanchez-Trincado J L, Gomez-Perosanz M, Reche P A. Fundamentals and methods for T‐and B‐cell epitope prediction. Journal of immunology research. 2017, 2017(1): 2680160. https://doi.org/10.1155/2017/2680160
- Chin S M, Kimberlin C R, Roe-Zurz Z, et al. Structure of the 4-1BB/4-1BBL complex and distinct binding and functional properties of utomilumab and urelumab. Nature Communications. 2018, 9(1): 4679. https://doi.org/10.1038/s41467-018-07136-7
- Hao Y, Yu X, Bai Y, et al. Cryo-EM Structure of HER2-trastuzumab-pertuzumab complex. PLoS One. 2019, 14(5): e0216095. https://doi.org/10.1371/journal.pone.0216095
- Hamed S M, Sakr M M, El-Housseiny G S, et al. State of the art in epitope mapping and opportunities in COVID-19. Future science OA. 2023, 9(1): FSO832. https://doi.org/10.2144/fsoa-2022-0048
- Tang X, Zhang W, Zhang Z. Developing T Cell Epitope-Based Vaccines Against Infection: Challenging but Worthwhile. Vaccines. 2025, 13(2): 135. https://doi.org/10.3390/vaccines13020135