Creative Biostructure provides tailored Mempro™ protein production services for CRAL-TRIO domain-containing proteins using cell-free expression system. Our cell-free expression systems can best preserve the native activity of CRAL-TRIO domain-containing proteins.
Mempro™ cell-free protein production systems are the innovative platform to produce integral membrane proteins, which can bypass the limitations that encountered in cell-based protein production systems, such as host cell toxicity, low yield and solubilization and purification using detergents. CRAL-TRIO domain is a structural domain that forms a hydrophobic binding pocket to bind small lipophilic ligands. The CRAL-TRIO domain contains several alpha helices as well as a beta sheet composed of 6 strands. Strands 2,3,4 and 5 form a parallel beta sheet with strands 1 and 6 being anti-parallel. CRAL-TRIO domain is found in GTPase-activating proteins (GAPs), a guanine nucleotide exchange factors (GEFs) and a family of hydrophobic ligand binding proteins, including the yeast SEC14 protein.
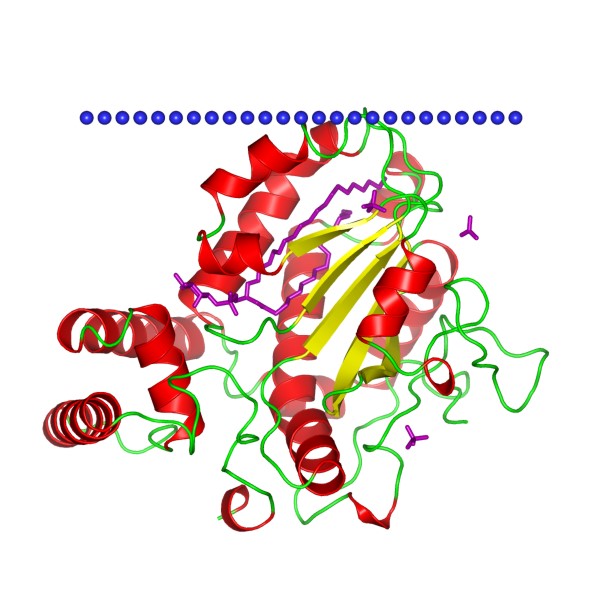
Figure 1. The structural model of yeast Sec14 homolog Sfh1. (OPM database)
Creative Biostructure has entensive in high-yield CRAL-TRIO domain-containing protein production using cell-free protein expression systems, we can provide various strategies for Mempro™ cell-free protein production, including:
- Mempro™ Cell-Free Protein Production in E. coli;
- Mempro™ Cell-Free Protein Production in Wheat Germ;
- Mempro™ Cell-Free Protein Production in Rabbit Reticulocyte;
- Mempro™ Cell-Free Protein Production in Baculovirus.
Among Mempro™ cell-free membrane protein production systems, E. coli S30 extract is commonly used for CRAL-TRIO domain-containing protein production. Wheat germ extract can provide eukaryotic translation system for CRAL-TRIO domain-containing protein expression. Baculovirus expression system from Autographa californica nucleopolyhedrovirus (AcNPV) is also a major system for CRAL-TRIO domain-containing protein expression, which is developed in the early stage that transfected insect cells in combination with vectors derived from the baculovirus. Crude rabbit reticulocyte lysate is also the most widely used for eukaryotic cell-free membrane protein expression. To employ an appropriate source of the extract, many factors, such as the availability of materials, protein origin and complexity, downstream processing, etc., should be considered.
With the Mempro™ cell-free protein production platform, Creative Biostructure is capable of expressing, purifying and crystallizing CRAL-TRIO domain-containing proteins to facilitate the study of their biological functions.
Most notably, One challenge associated with Mempro™ cell-free membrane protein production is that degradation of the DNA by endogenous nucleases. Creative Biostructure can optimize Mempro™ cell-free membrane protein production for CRAL-TRIO domain-containing proteins using plasmids owing to they are circular and have no end for the exonucleases to attach.
We provide other various Mempro™ membrane protein production services. Please feel free to contact us for a detailed quote.
References:
Cell-free protein synthesis. (https://en.wikipedia.org/wiki/Cell-free_protein_synthesis)
F. Bernhard, et al. (2013). Cell-free expression - making a mark. Cur. Opin. Struct. Biol., 23: 374-380.
J. M. bomar, et al. (2003). Mutations in a novel gene encoding a CRAL-TRIO domain cause human Cayman ataxia and ataxia/dystonia in the jittery mouse. Nature Gen., 35: 264-269.
K. G. Johnson and K. Kornfeld (2010). The CRAL/TRIO and GOLD domain protein TAP-1 regulates RAF-1 activation. Dev. Biol., 341(2): 464-471.
