Viral Envelope Glycoprotein Crystallization
With the most advanced technology and years of experience in the protein crystallization field, Creative Biostructure now provides advanced custom Mempro™ envelope glycoprotein crystallization services, covering all experimental steps from gene to structure with an emphasis on protein purification, crystallization, structure determination and analysis. Our aim is to help customers accelerate research progress in a highly productive and cost-effective way.
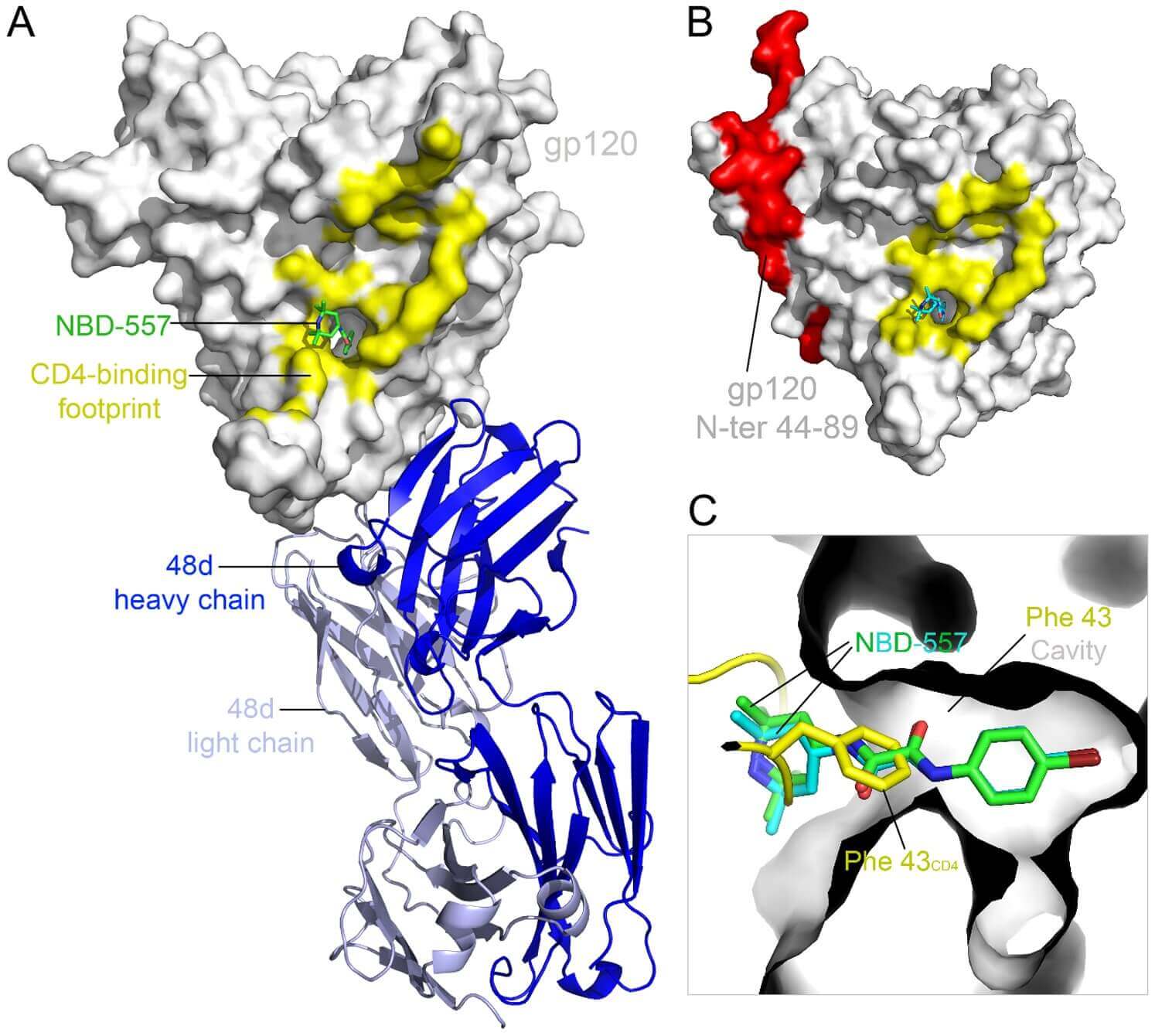
Figure 1. Structures of HIV-1 gp120 core in complex with NBD-557.
Entry of enveloped viruses into their host cells requires fusion of the viral and cellular membranes, a process that is mediated by the viral envelope glycoprotein. Class I viral fusion proteins, such as influenza and human immunodeficiency virus type 1 (HIV-1), are synthesized as inactive precursor glycoproteins that assemble as trimers and are subsequently primed by proteolytic cleavage to generate the mature fusogenic spikes. These spikes on the virion surface, primed to undergo large-scale conformational changes culminating in virus-cell membrane fusion and viral entry. Therefore, the prefusion form of these envelope glycoproteins represents an important molecular target for antiviral intervention, which provides a sound basis for vaccine and drug development.
Because of the important roles of viral envelope glycoproteins in receptor binding and interactions with neutralizing antibodies, it is important to get their structural information for understanding virus infection and designing therapeutic and prophylactic strategies. However, a critical roadblock has always been the inability to produce the envelope glycoprotein trimer. X-ray crystallographic analyses of the most extensively characterized class I envelope glycoproteins – influenza virus hemagglutinin (HA), HIV-1 envelope glycoprotein (Env) and parainfluenza virus 5 F (PIV5 F) – are based on soluble ectodomain fragments. The failure of current HIV-1 vaccines to elicit broadly neutralizing antibodies is largely attributed to the inability to produce the trimeric native Env in a prefusion conformation. Creative Biostructure has a professional team experienced in the production of viral envelope glycoproteins while maintaining the prefusion state as well as the activated state with fusogenic conformational changes. Crystallization conditions are then screened in a high-throughput manner on our X-ray Crystallography Platform.
Following optimized expression, purification and crystallization of your protein(s) of interest, Creative Biostructure guarantees precise determination of the native target structure(s) with medium or high resolution. Based on structural analysis, we also provide a series of downstream services such as antibody development, drug discovery and vaccine formulation development to meet your demands.
Please feel free to contact us to discuss your project!
Ordering Process
References
- Kwon YD, et al. (2014) “Crystal structures of HIV-1 gp120 envelope glycoprotein in complex with NBD analogues that target the CD4-binding site. PLoS One 9(1):e85940.
- Harrison SC. (2008) “Viral membrane fusion”. Nat Struct Mol Biol 15:690-698.
- White JM, et al. (2008) “Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme”. Crit Rev Biochem Mol Biol 43:189-219.
- Burton DR, et al. (2004) “HIV vaccine design and the neutralizing antibody problem”. Nat Immunol 5:233-236.
