Rheo-NMR Service
Natural structural biomolecules such as silk proteins and collagen exhibit unique properties that are closely related to the structure of the aggregated state of their molecular chains. Shear stress can cause structural deformation of proteins, which may lead to the formation of aggregates and thus affect the intermolecular binding and interactions of proteins. Therefore, researchers not only need to understand the relationship between the structure and properties of structural protein molecular chains but also need to study the mechanisms of changes in these aggregate-state structures in organisms in a comprehensive and in-depth manner.
Given that in most cases the aggregated state structures of proteins are generated under dynamic conditions, Creative Biostructure offers Rheo-NMR spectroscopy service to researchers to assist them in studying the changes in the aggregation profiles of structural protein molecular chains under shear-induced effects.
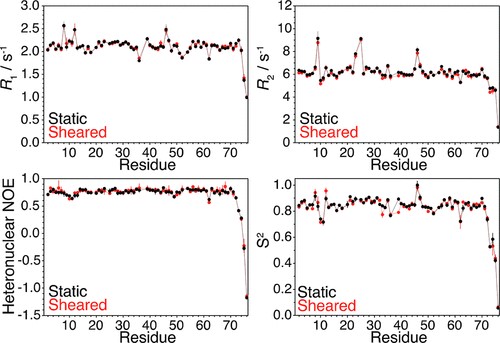 Figure 1. Relaxation data obtained by high-sensitivity Rheo-NMR. (Daichi, M.; et al. 2017)
Figure 1. Relaxation data obtained by high-sensitivity Rheo-NMR. (Daichi, M.; et al. 2017)
- It is beneficial for our customers to explore and establish methods to study the structural and molecular dynamics of structural proteins under dynamic conditions.
- It can provide our customers with a comprehensive understanding of the mechanism of structural protein aggregation behavior in solution and its control conditions, establish a reasonable theoretical model, and provide technical support for the preparation of high-performance bionanomaterials.
- The detailed rheological response of aggregation-prone proteins can be revealed, which helps to elucidate the mechanism of pathological aggregates and protofibril formation, thus enabling our customers to better understand the pathogenesis of relevant diseases.
General Process
- Rheo-NMR instrument construction
- NMR measurement and analysis
- Spatial-temporal decomposition of intrinsic NMR parameters (e.g. relaxation time) or motions (e.g. diffusion or flow)
Technical Advantages
- Compatible with cryogenic probes (cryoprobes)
- Enable protein NMR measurements with high sensitivity
- Enable capture of aggregate nucleation and subsequent growth at atomic resolution
- Obtain high-quality structural information that is difficult to obtain by other methods
- Enable quantification of the number of proteins in their natural state
Obtaining high-quality data on protein samples will help to understand the molecular behavior of proteins under directional stress. Rheo-NMR spectroscopy has the potential to monitor structural changes in proteins under shear stress at the atomic level and is the method of choice for studying the behavior of macromolecules under unidirectional stress at atomic resolution. Customers who have a need or any questions regarding the Rheo-NMR spectroscopy technical service offered by Creative Biostructure are welcome to contact us at any time. We look forward to working with you on your next groundbreaking project.
Ordering Process
Reference
- Morimoto D.; et al. High-sensitivity Rheo-NMR spectroscopy for protein studies. Analytical Chemistry. 2017, 89(14): 7286-7290.
