X-Ray Free Electron Laser (XFEL) Service
Biological macromolecules are the basis of all life activities, and their structure and functional studies have been a major frontier topic in life science research. X-ray crystallography is the most important method to study the three-dimensional structures of biological macromolecules. Currently, nearly 89% of the protein structures in the Protein Data Bank are obtained by synchrotron X-ray sources, but the radiation damage of X-rays limits the application of X-ray crystallography in protein structure and dynamics studies.
X-ray free-electron lasers (XFEL) use free electrons as a medium to generate X-ray pulses with very high peak intensities and ultra-short pulse durations to break the link between radiation damage, sample size, and resolution, which passes through the sample before the X-ray damage. XFEL can obtain high-resolution protein structures and electronic state information. It can be used for both protein nanocrystals and giant virus particles, providing new opportunities for protein structure and dynamics studies.
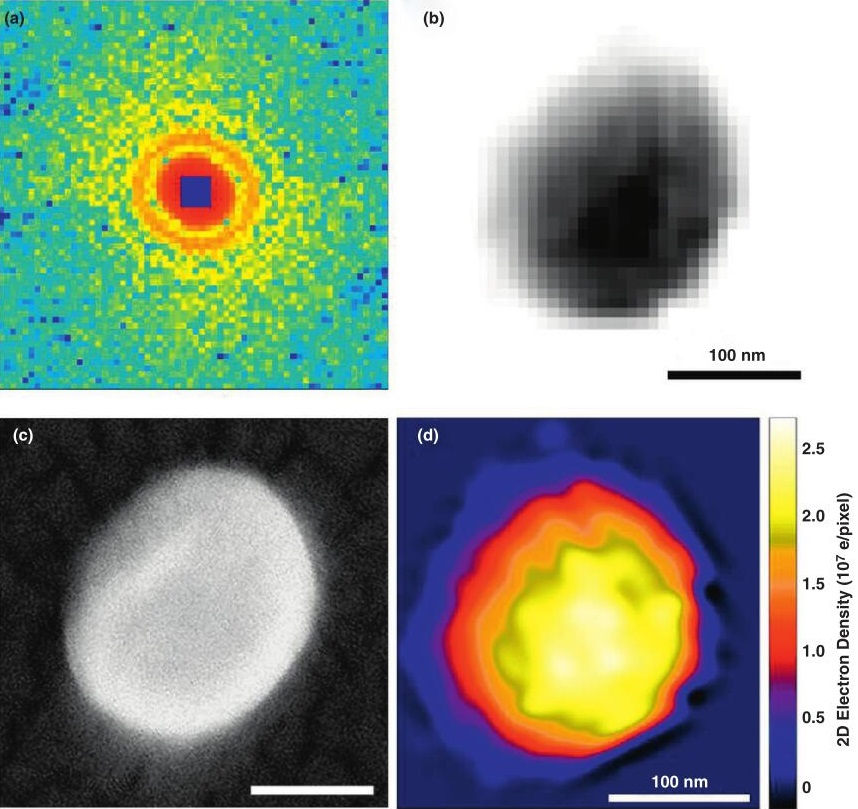 Figure 1. Single particle coherent diffraction imaging of herpesvirus virions. (Ilme, S, et al., 2012)
Figure 1. Single particle coherent diffraction imaging of herpesvirus virions. (Ilme, S, et al., 2012)
The emergence of XFEL has greatly promoted the development and application of coherent diffraction imaging. Depending on the state of the sample and the experimental setup, XEFL imaging techniques are generally divided into serial crystal diffraction imaging (SFX) and single-particle imaging (SPI) with the ability to resolve tiny protein crystals.
XFEL offers the advantages of both X-rays and lasers, with peak brightness 9 orders of magnitude higher, pulse width 3 orders of magnitude shorter, and coherence more than 3 orders of magnitude higher than typical third-generation synchrotron radiation sources.
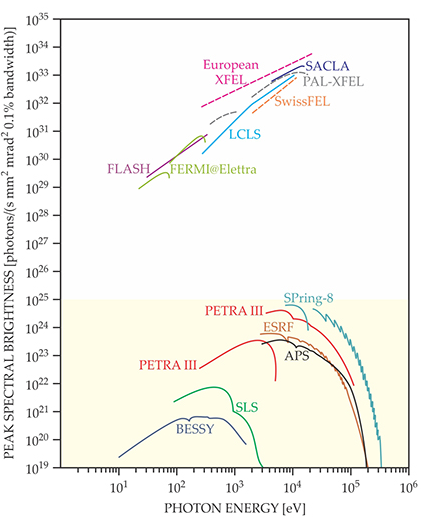 Figure 2. The peak brightnesses of select free-electron lasers (FELs) and synchrotron facilities are shown for a wide photon energy range. (Philip, H, B, et al., 2015)
Figure 2. The peak brightnesses of select free-electron lasers (FELs) and synchrotron facilities are shown for a wide photon energy range. (Philip, H, B, et al., 2015)
XFEL offers new opportunities for structural analysis of difficult targets, provides feasibility for determining new protein structures, and is an important tool for determining the structure of difficult-to-crystallize proteins, such as membrane proteins.
Creative Biostructure XFEL service not only greatly improves the efficiency of existing experimental methods, but also makes some new experimental protocols possible, which is expected to help our customers produce more impactful new results and spawn a series of novel phenomena. Please feel free to contact us. We are looking forward to cooperating with you.
Ordering process
References
- Ilme, S, et al. Emerging opportunities in structural biology with X-ray free-electron lasers. Current Opinion in Structural Biology. 2012, 22(5): 613-626.
- Philip, H, B, et al. Brighter and faster: The promise and challenge of the x-ray free-electron laser. Physics Today. 2015, 68(7): 26-32.
