Nuclear Magnetic Resonance (NMR) spectroscopy is one of the most powerful analytical techniques in organic chemistry. Not only does it elucidate molecular structures with remarkable detail, but it also plays a crucial role in distinguishing between isomers—compounds with the same molecular formula but different structural or spatial arrangements. The ability of NMR to probe the electronic environments of nuclei provides chemists with rich, nuanced data that can reveal subtle differences in molecular architecture.
Isomerism, a fundamental concept in organic chemistry, includes structural isomerism (constitutional isomers) and stereoisomerism (encompassing geometric and optical isomers). Identifying these isomers is essential in areas such as drug development, natural product synthesis, and chemical manufacturing, where slight structural variations can lead to dramatically different properties and biological activities.
Creative Biostructure provides high-quality, customized NMR spectroscopy services tailored to the precise identification and differentiation of isomers in organic chemistry, helping researchers unlock structural insights with confidence and accuracy.
 Figure 1. An example of 1H-NMR spectrum of isomers 1-E,E and 1-E,Z in CDCl3; and 1-Z,Z in acetone-d6. (Gordillo et al., 2017)
Figure 1. An example of 1H-NMR spectrum of isomers 1-E,E and 1-E,Z in CDCl3; and 1-Z,Z in acetone-d6. (Gordillo et al., 2017)
Principles of NMR Spectroscopy
NMR spectroscopy is based on the absorption of radiofrequency radiation by nuclei in a magnetic field. Most commonly, 1H and 13C nuclei are studied. When placed in a magnetic field, these nuclei align with or against the field, creating energy states. Transition between these states upon absorption of energy is detected and translated into a spectrum.
The exact frequency at which a nucleus resonates depends on its electronic environment, known as the chemical shift. Additionally, interactions between nearby nuclei (spin-spin coupling) create signal multiplicities that reveal more about molecular structure. These features make NMR uniquely suited for distinguishing between isomers.
Structural (Constitutional) Isomers
Constitutional isomers differ in the connectivity of atoms. NMR can reveal these differences via distinct chemical shifts, different integration patterns, and coupling information.
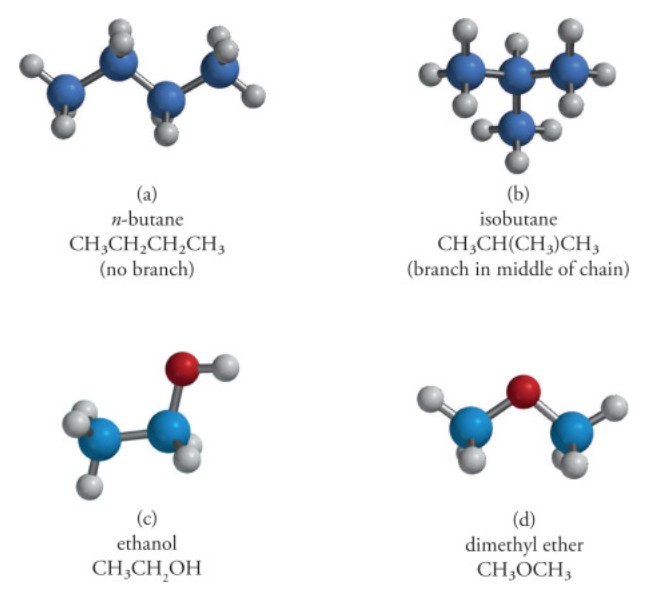 Figure 2. Structures of constitutional isomers. Constitutional isomers have the same molecular formula but different connectivity. Examples of constitutional isomers include n-butane and isobutane, as well as ethanol and dimethyl ether.(Ouellette and Rawn, 2018)
Figure 2. Structures of constitutional isomers. Constitutional isomers have the same molecular formula but different connectivity. Examples of constitutional isomers include n-butane and isobutane, as well as ethanol and dimethyl ether.(Ouellette and Rawn, 2018)
Chemical Shifts and Signal Count
Each unique proton or carbon environment gives rise to a separate signal in NMR. For example, consider the isomers C3H8O:
- Propan-1-ol: CH3-CH2-CH2OH
- Propan-2-ol: (CH3)2CHOH
Their 1H NMR spectra are markedly different. Propan-1-ol has three distinct proton environments (OH, CH2, CH3), while propan-2-ol exhibits different splitting patterns and chemical shifts due to the branching. The OH proton in both cases may appear as a singlet, but its position varies based on hydrogen bonding.
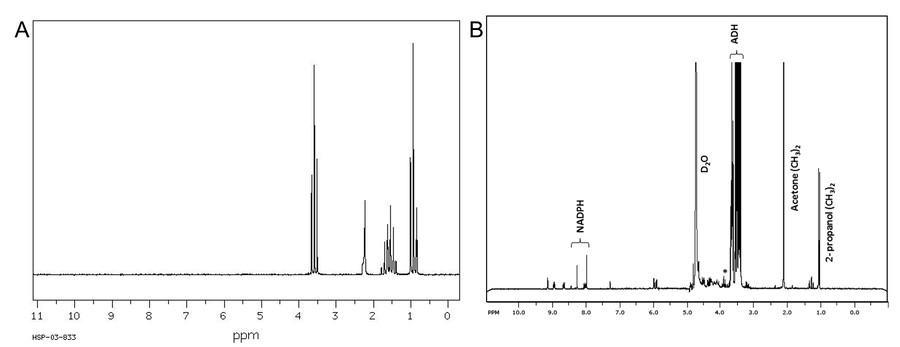 Figure 3. A. 1H NMR spectrum of propan-1-ol in CDCl3, 90MHz. (chemicalbook.com) B. 1H NMR spectrum of propan-2-ol doublet peak at 1 ppm) produced by the reduction of acetone, substrate concentration 50 mM acetone in D2O/100 mM Na phosphate, pH 7.2 buffer. Chemical shifts of the single and hydroxyl proton of 2-propanol are indicated by an asterisk. (Leitsch et al., 2013)
Figure 3. A. 1H NMR spectrum of propan-1-ol in CDCl3, 90MHz. (chemicalbook.com) B. 1H NMR spectrum of propan-2-ol doublet peak at 1 ppm) produced by the reduction of acetone, substrate concentration 50 mM acetone in D2O/100 mM Na phosphate, pH 7.2 buffer. Chemical shifts of the single and hydroxyl proton of 2-propanol are indicated by an asterisk. (Leitsch et al., 2013)
13C NMR
Carbon NMR often provides a clearer distinction among constitutional isomers, particularly when proton environments are ambiguous. The number and type of carbon signals differ, as in the case of:
- n-butane: CH3CH2CH2CH3 (2 unique carbon types)
- isobutane: (CH3) 3CH (2 unique carbon types, but with different symmetry)
The symmetry and environment of each carbon lead to different chemical shift values.
Stereoisomers
Stereoisomers have the same connectivity but differ in spatial arrangement. These include geometric isomers (cis/trans or E/Z) and optical isomers (enantiomers and diastereomers).
Geometric Isomers: Cis/Trans or E/Z
Alkenes and cyclic compounds often exhibit cis-trans isomerism. NMR can distinguish these through:
- Coupling constants (J values): Vicinal coupling constants in alkenes provide a clear indication. Trans hydrogens usually show J = 12-18 Hz, while cis hydrogens exhibit J = 6-12 Hz.
- Chemical shifts: Spatial proximity affects shielding. In cis isomers, protons may be deshielded due to anisotropic effects of nearby groups.
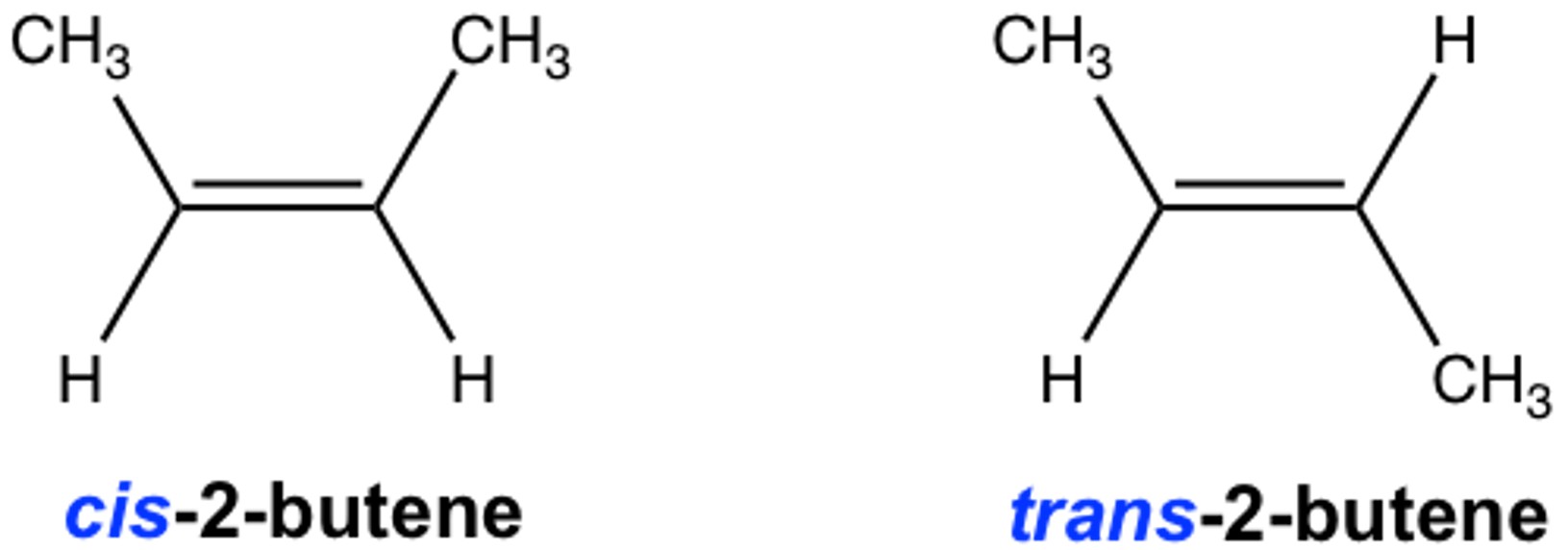 Figure 4. Geometric isomers of disubstituted cycloalkanes.
Figure 4. Geometric isomers of disubstituted cycloalkanes.
Diastereomers
Diastereomers are non-mirror image stereoisomers. They have different physical and chemical properties, and their NMR spectra are distinct.
For example, consider the threonine stereoisomers. The alpha and beta protons in different stereoisomers show different chemical shifts and coupling patterns due to variations in spatial orientation. This is further exaggerated in chiral solvents or when derivatized with chiral auxiliaries.
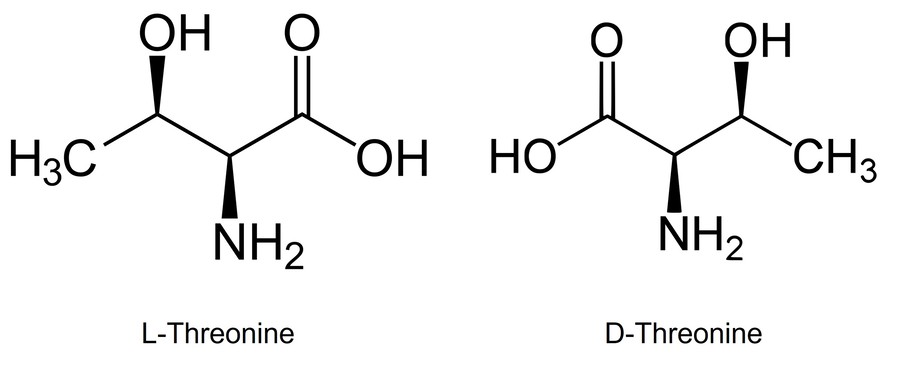 Figure 5. Threonine stereoisomers.
Figure 5. Threonine stereoisomers.
Enantiomers
Enantiomers, being mirror images, have identical NMR spectra in achiral environments. However, they can be distinguished by:
- Chiral Solvating Agents (CSAs): Compounds like Eu(hfc)3 or (R)-binol create diastereomeric complexes with each enantiomer, shifting their signals differently.
- Chiral Derivatizing Agents (CDAs): Mosher's acid (MTPA) forms diastereomers upon reaction, enabling discrimination.
- Chiral stationary phase NMR techniques: Emerging methods allow for the direct differentiation of enantiomers through NMR.
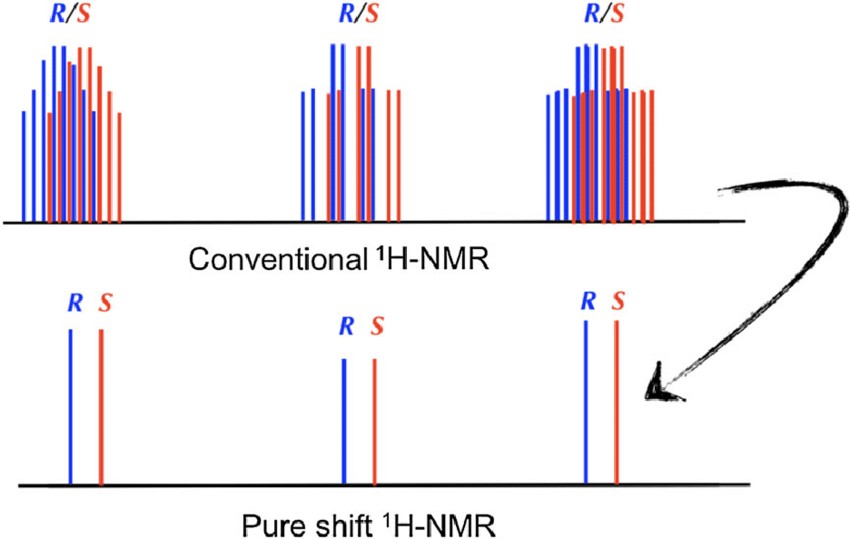 Figure 6. Cartoon representation of 1H-NMR spectra of enantiomers, upper trace represents conventional 1H-NMR and lower trace the pure shift NMR spectra. (Nath et al., 2018)
Figure 6. Cartoon representation of 1H-NMR spectra of enantiomers, upper trace represents conventional 1H-NMR and lower trace the pure shift NMR spectra. (Nath et al., 2018)
Advanced NMR Techniques for Isomer Differentiation
| Techniques | Details |
|---|---|
| 2D NMR Techniques |
|
| NOESY and ROESY | These techniques detect nuclear Overhauser effects, which arise from spatial proximity (<5 Å). Particularly useful for stereoisomers, NOE can confirm cis/trans arrangements or relative stereochemistry. |
| Variable Temperature NMR | Dynamic isomerism, such as ring flipping or amide bond rotation, can be probed using temperature-dependent NMR. Lowering the temperature often "freezes" conformers, making their signals distinct. |
Applications of NMR in Identifying Isomers
- Drug Isomers: Many drugs have active and inactive enantiomers. Thalidomide's tragic history highlights the importance of enantiomer identification. Modern drug development rigorously employs NMR for isomer purity.
- Reaction Monitoring: Tracks isomerization reactions in real time, helping chemists understand how and when isomers form or convert.
- Chiral Analysis: Combined with chiral solvating agents, NMR can distinguish enantiomers—super valuable in drug development and asymmetric synthesis.
- Conformational Studies: Reveals how a molecule's 3D shape changes in different environments, especially important for bioactive molecules.
- Natural Product Analysis: Dissects complex mixtures of isomers in extracts from plants, microbes, etc.
Select Service
Limitations and Complementary Techniques
While NMR is immensely powerful, it is not without limits:
- Enantiomers are invisible without chiral agents.
- Signal overlap in complex mixtures can complicate analysis.
- Low sensitivity of 13C and other nuclei may require long acquisition times.
Complementary techniques include:
- Mass spectrometry (MS): Offers molecular weight and fragmentation data.
- Infrared spectroscopy (IR): Useful for identifying functional groups.
- X-ray crystallography: Gold standard for absolute stereochemistry when crystals are available.
Case Studies
Case 1: Synthesis of aryl(alkyl)azole oximes: E/Z ratio via 1H NMR and HOMO–LUMO analysis
In this study, 12 oxime derivatives were synthesized using both conventional and microwave irradiation methods to compare their efficiency. Microwave-assisted synthesis yielded higher product quantities. Due to the presence of C=N double bonds, these oxime compounds exhibit E/Z geometric isomerism. The E/Z ratios were analyzed, revealing that pyrazole derivatives predominantly formed Z-isomers, while imidazole derivatives synthesized via the conventional method mostly yielded E-isomers. Structural confirmation was conducted using IR, 1H-NMR, 13C-NMR, and HRMS techniques. Additionally, Density Functional Theory (DFT) calculations were used to evaluate HOMO-LUMO energies and thermodynamic properties in different environments (water, ethanol, vacuum). Parameters such as chemical hardness, potential, electrophilicity, and softness were also calculated based on these energies.
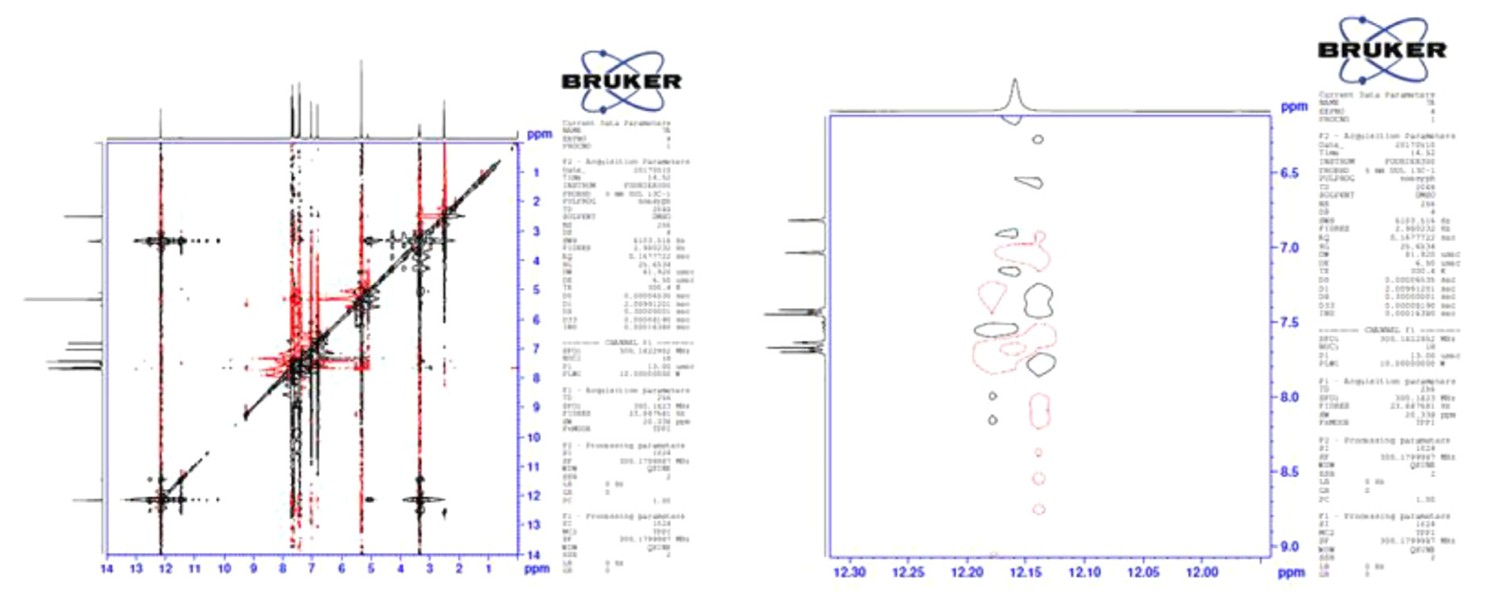 Figure 7. Two-dimensional nuclear magnetic resonance (2D-NMR) of D4 spectra. (Bozbey et al., 2021)
Figure 7. Two-dimensional nuclear magnetic resonance (2D-NMR) of D4 spectra. (Bozbey et al., 2021)
Case 2: Solid-state photo-NMR study on light-induced nitrosyl linkage isomers
This study reports the first solid-state MAS NMR detection and characterization of a light-induced NO linkage isomer (MS1) in trans-Ru(15NO)(py)419F2. Using NMR, researchers measured 15N and 19F chemical shift tensors, spin-spin couplings, and T1 relaxation times at temperatures below 250 K. Two crystallographically distinct cation sites allowed for separate evaluation of MS1 populations and thermal decay.
The study revealed that MS1 is diamagnetic, evidenced by its shorter T1 relaxation times compared to the ground state (GS). Upon light exposure at 420 nm, the NO ligand undergoes a ~180° rotation, which results in an increase in the isotropic chemical shift of 15N and a corresponding decrease in that of 19F. Additionally, the spin-spin coupling between 15N and 19F increases significantly, from 71 Hz in the ground state to 105 Hz in the MS1 state.
This work provides the first experimental evidence of diamagnetism in a photo-induced NO isomer of a ruthenium complex and offers insights into structural and electronic changes upon photoisomerization.
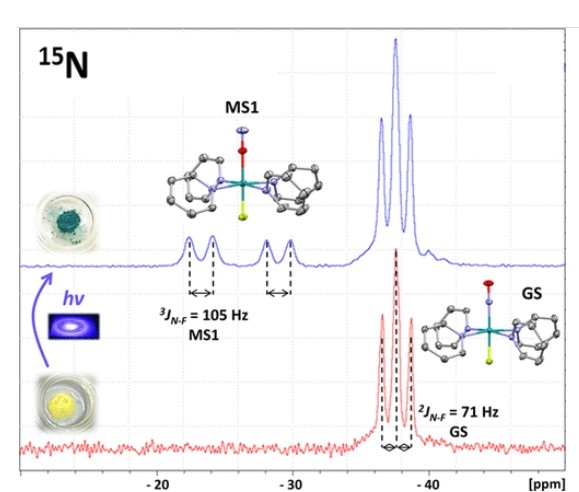 Figure 8. NMR spectrum of light-induced NO linkage isomer (MS1). (Gansmüller et al., 2022)
Figure 8. NMR spectrum of light-induced NO linkage isomer (MS1). (Gansmüller et al., 2022)
In summary, NMR spectroscopy remains an indispensable tool in the identification of isomers in organic chemistry. Its ability to detect minute differences in chemical environment and spatial arrangement makes it uniquely suited for this task. Through a combination of chemical shifts, coupling constants, NOE interactions, and multidimensional correlation experiments, chemists can confidently distinguish between structural, geometric, and optical isomers.
As the complexity of synthetic targets and the demand for stereochemical purity in pharmaceuticals increase, the role of NMR will only become more central. With ongoing advancements in instrumentation, pulse sequences, and chiral methodologies, the future of isomer analysis via NMR looks brighter than ever.
At Creative Biostructure, we offer expert NMR spectroscopy services tailored to isomer identification and beyond. Contact us today to discuss your project—we're here to help you see the unseen.
References
- Bozbey I, Uslu H, Türkmenoğlu B, Özdemir Z, Karakurt A, Levent S. Conventional and microwave prompted synthesis of aryl(Alkyl)azole oximes, 1H-NMR spectroscopic determination of E/Z isomer ratio and HOMO-LUMO analysis. Journal of Molecular Structure. 2022;1251:132077.
- Gansmüller A, Mikhailov AA, Kostin GA, et al. Solid-state photo-NMR study on light-induced nitrosyl linkage isomers uncovers their structural, electronic, and diamagnetic nature. Anal Chem. 2022;94(10):4474-4483.
- Gordillo MA, Soto‐Monsalve M, Carmona‐Vargas CC, et al. Photochemical and electrochemical triggered bis(Hydrazone) switch. Chemistry A European J. 2017;23(59):14872-14882.
- Leitsch D, Williams CF, Lloyd D, Duchêne M. Unexpected properties of NADP-dependent secondary alcohol dehydrogenase (ADH-1) in Trichomonas vaginalis and other microaerophilic parasites. Experimental Parasitology. 2013;134(3):374-380.
- Nath N, Bordoloi P, Barman B, Baishya B, Chaudhari SR. Insight into old and new pure shift nuclear magnetic resonance methods for enantiodiscrimination. Magnetic Reson in Chemistry. 2018;56(10):876-892.
- Ouellette RJ, Rawn JD. Functional groups and their properties. In: Organic Chemistry. Elsevier; 2018:31-49.