Structural Research of Sterol Isomerases
Sterol Isomerases, also known as emopamil binding proteins (EBPs), are endoplasmic reticulum membrane proteins involved in cholesterol biosynthesis, autophagy, and oligodendrocyte formation. Recently, research has shown that some EBP ligands have been shown to cause cancer cell death by affecting cholesterol metabolism. Therefore, it is important to discover critical residues for function by structurally resolving EBP to develop new approaches for cancer therapy.
Action mechanism of human EBP
Human sterol Δ8-Δ7 isomerase is involved in the post-squalene pathway of cholesterol biosynthesis. The isomerase transfers the double bond from the C8-C9 position of the sterol or enol to the C7-C8 position of the sterol B ring to produce 24-dehydrolathosterol or lathosterol. Notably, EBP binds a large number of pharmacologically active compounds with diverse structures, including antidepressants, antipsychotics, opioid analgesics, sterol biosynthesis inhibitors, and antitumor reagents. As a component of the microsomal anti-estrogen binding site (AEBS), EBP is involved in the estrogen receptor-independent action of tamoxifen, which can reduce intracellular tamoxifen utilization and lead to drug resistance.
Overall Structure of the EBP Protein
The researchers resolved two crystal structures of EBP complexed with tamoxifen and U18666A by selenium-based single-wavelength anomalous dispersion (SAD) at 3.5 Å and 3.2 Å resolution. The structures reveal that the protein contains five transmembrane helices (TMs 1-5). the linker between TM2 and TM3 forms a horizontal helix at the membrane level (H1), and the residues facing the cavity of H1 are hydrophobic and aromatic, whereas the residues facing the lumen are hydrophilic. The protein structure contains critical catalytic residues (His 76, Glu 80, Glu 122, and Trp 196).
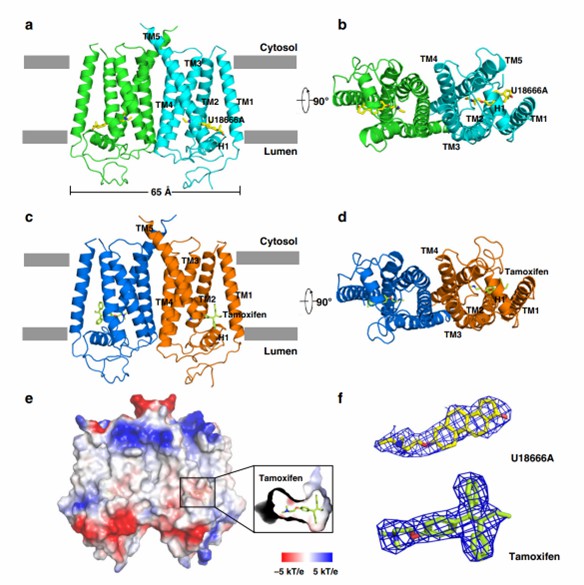 Figure 1. Molecular architecture of human EBP. (Long, T., et al., 2019)
Figure 1. Molecular architecture of human EBP. (Long, T., et al., 2019)
| Protein | Organism | Method | Resolution | PDB Entry ID |
| EBP and U18666A | Homo sapiens | X-ray diffraction | 3.2 Å | 6OHT |
| EBP and tamoxifen | Homo sapiens | X-ray diffraction | 3.526 Å | 6OHU |
| RV0760c | Mycobacterium tuberculosis H37Rv | X-ray diffraction | 2.1 Å | 2Z7A |
| RV0760c | Mycobacterium tuberculosis H37Rv | X-ray diffraction | 1.82 Å | 2Z76 |
| RV0760c in complex with estradiol-17beta-hemisuccinate | Mycobacterium tuberculosis H37Rv | X-ray diffraction | 2.03 Å | 2Z77 |
| Isopentenyl diphosphate isomerase | Homo sapiens | X-ray diffraction | 1.7 Å | 2ICJ |
| Isopentenyl diphosphate isomerase complexed with substrate analog | Homo sapiens | X-ray diffraction | 1.93 Å | 2ICK |
| IPP isomerase I in space group P212121 | Homo sapiens | X-ray diffraction | 1.6 Å | 2DHO |
| Mycothiol-dependent maleylpyruvate isomerase | Corynebacterium glutamicum | X-ray diffraction | 1.75 Å | 2NSF |
| OSC in complex with Ro 48-8071 | Homo sapiens | X-ray diffraction | 2.2 Å | 1W6J |
| OSC in complex with Lanosterol | Homo sapiens | X-ray diffraction | 2.1 Å | 1W6K |
| Mevalonate 5-diphosphate decarboxylase and isopentenyl diphosphate isomerase | Escherichia coli | X-ray diffraction | 2.5 Å | 1I9A |
| Isopentenyl pyrophosphate-dimethylallylpyrophosphate isomerase | Escherichia coli | X-ray diffraction | 1.96 Å | 1NFS |
Table 1. Structural research of the sterol isomerases.
With years of experience in the structure inspection industry, Creative Biostructure has become a well-known and reliable partner in membrane protein structure analysis services. Our experienced scientists utilize cutting-edge techniques including X-ray crystallography, cryo-electron microscopy (cryo-EM), and nuclear magnetic resonance (NMR) spectroscopy to obtain high-resolution structural data on sterol isomerases. If you require structural testing of proteins and other compounds, please contact us for a customized experimental solution that will advance your scientific goals.
References
- Long, T., et al. Structural basis for human sterol isomerase in cholesterol biosynthesis and multidrug recognition. Nature Communications. 2019. 10: 2452.
- Yao H, et al. Thermostabilization of Membrane Proteins by Consensus Mutation: A Case Study for a Fungal Δ8-7 Sterol Isomerase. J Mol Biol. 2020. 432(18): 5162-5183.
- Cai H, et al. High-level heterologous expression of the human transmembrane sterol Δ8, Δ7-isomerase in Pichia pastoris. Protein Expr Purif. 2019. 164: 105463.